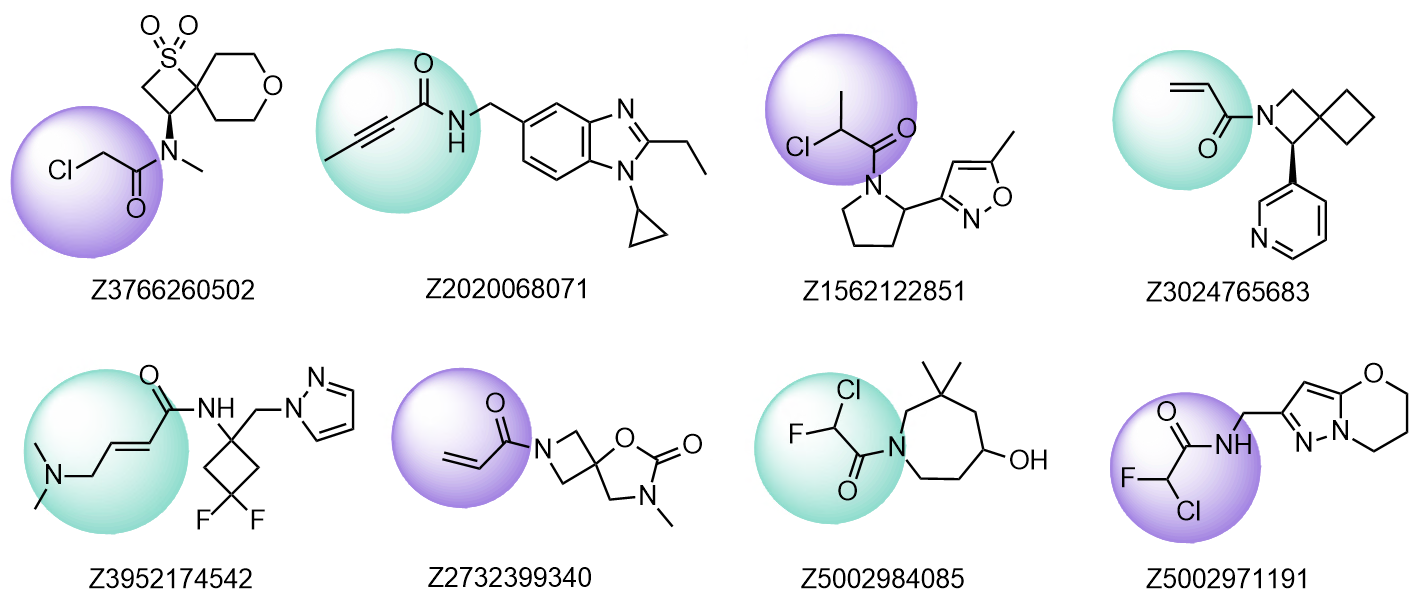
Library of Cys-specific covalent electrophilic binders
3 200 compounds
Covalent chemical probes become an important tool in drug discovery within last few years. The impressive number of successful applications in protein drugability assessment, especially focusing on Cys residue, brought aspiration to discovery and synthesis of new covalent modifiers.
We used deep knowledge-based approach to design and synthesize our cysteine focused fragment library. Careful choice of covalent warheads based on their reactivity was performed by experienced chemists and reflected a small set of specific structural filters. Electrophilic fragments selected by structural filters were then analyzed to remove any molecules with trivial or unwanted structural features. The resulting set was refined with Ro3 criteria applied to “core structures” to yield 3 200 fragments able to form covalent bonds with cysteine residues.
Hits derived from this library can be easily followed with analogues from stock or either synthesis of new compounds through REAL Database technology.
Typical Formats
Cysteine-Focused Covalent Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
CYS-3200-0-Z-10
Compounds
3 200
3 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
CYS-3200-10-Y-10
Compounds
3 200
10 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
CYS-3200-50-Y-10
Compounds
3 200
10 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, first and last two columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
CYS-3200-10-Y-10 screening library 3 200 cmpds, hit resupply, analogs from 4.7M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD file
Key features
- Only well-validated covalent warheads that form easy-to-interpret adducts.
- No overreactive, promiscuous binders.
- Attractive scaffolds, derived from the latest core building blocks.
- Plated at 100 mM concentration in DMSO.
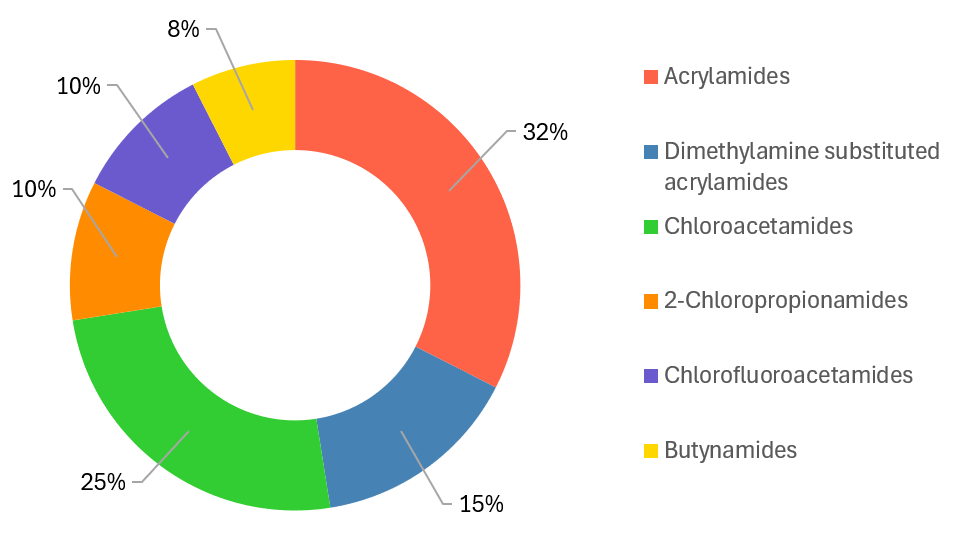
The following warheads were used for the library construction:
- Acrylamides – 1,040
- Dimethylamine substituted acrylamides – 480
- Chloroacetamides – 800
- 2-Chloropropionamides – 320
- Chlorofluoroacetamides – 320
- Butynamides - 240
Examples of the molecules in Cysteine focused Library