Designed for the discovery of new effective modulators of Wnt/β-catenin signaling pathway
10 560 compounds
The dysregulation of Wnt/β-catenin pathway is often a key reason for cancer and other serious diseases. Wnt signaling is a highly conserved pathway that controls all steps of embryo development1-6 and tissue maintenance in mature organisms8-11. Alzheimer's, multiple sclerosis (MS), polycystic kidney disease (PKD), type 2 diabetes, arthritis, colon cancer, lung and breast cancer are some examples of Wnt pathway dysfunction. Recent evidence suggests that activation of Wnt pathway may prevent osteogenesis17,30,31 and neurodegenerative disorders.18,32
To create the ultimate Wnt/β-catenin signaling modulators library, we focused on the essential and clinically relevant protein targets. We used all available structural information and activity data for the following targets: CK1α, GSK3α/β, frizzled (Fzd) receptors and Lrp co-receptors (Lrp5/6), zinc and ring finger proteins (Znrf3, Rnf43), DKK1, Dvl-Axin, β-Catenin/TCF4, β-Catenin/Bcl9 and Bcl9/PYGO/His3 interaction inhibitions and Tankyrase to assemble the library.
The library of potential Wnt signaling modulators has been pre-plated for the most convenient and quick access. Using our Wnt library you receive multiple benefits, allowing you to save on time and costs in hit exploration and follow-up optimization:
- Hit confirmation support: Analogs search and hit samples resupply from same batch with strict QC
- Straightforward & affordable synthesis of hit follow-up libraries by parallel chemistry
- Medicinal chemistry support enhanced with on-site broad ADME/T panel
Typical Formats
Catalog No.
WPL-10-0-Z-10
Compounds
10 560
9 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
WPL-10-10-Y-10
Compounds
10 560
33 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo LDV microplates #001-12782 (LP-0200), 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
WPL-10-50-Y-10
Compounds
10 560
33 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
*We will happily provide our library in any other most convenient format for your project. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or, Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400) or send your preferred labware. Compounds pooling can be provided upon request.
Download SD file
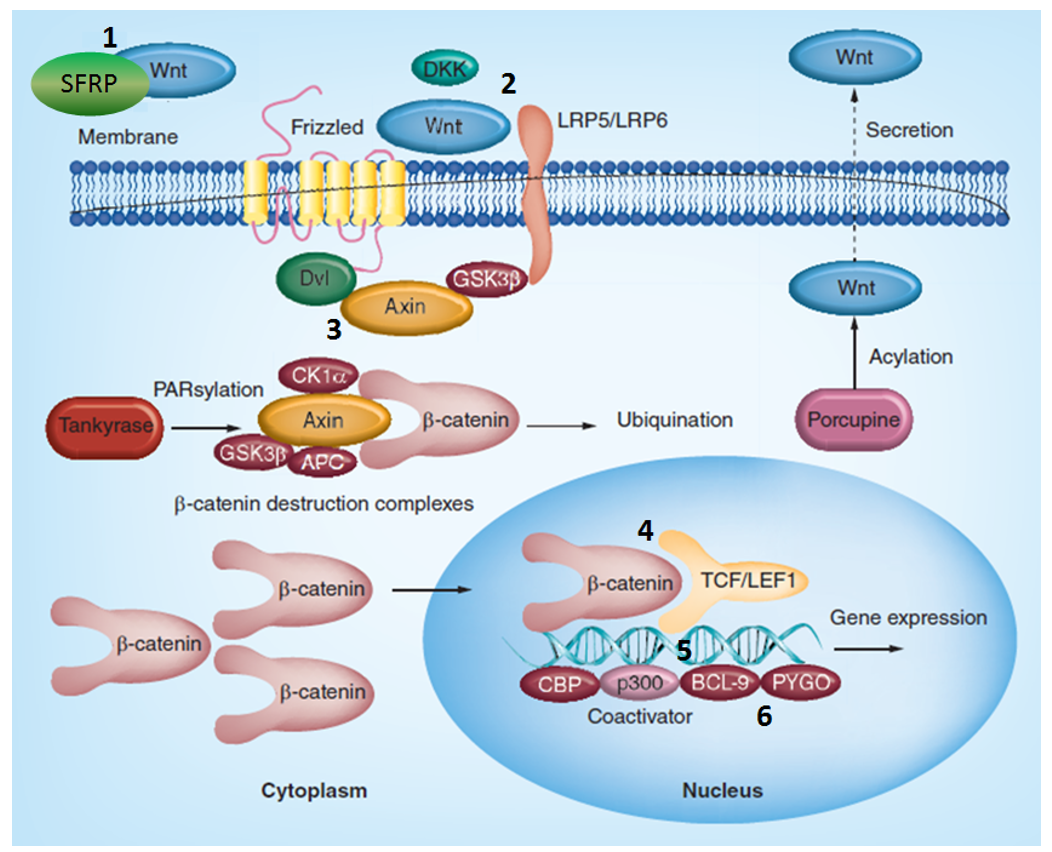
Figure 1. Canonical Wnt/Fz signaling cascade (adopted from Future Med. Chem. (2015), 7(18), 2485–2505) showing specific target-biased focused libraries available from Enamine.
Whereas numerous publications describe both identification and clinical development of Wnt inhibitors as potential anti-tumor therapies,19,20 limited number of reports deal with the therapeutic utility of Wnt activators and/or potentiators.21 These classes of small molecule modulators usually inhibit kinases implicated in stability of β-catenin, namely GSK3β and CK1. For example, CHIR-99021 (CT99021) is a GSK-3α/βinhibitor with IC50 of 10 nM/6.7 nM and > 500-fold selectivity for GSK-3 versus its closest homologs CDC2 and ERK2, as well as other protein kinases. Administration of CHIR-99021 significantly augmented hematopoietic repopulation in recipient mice transplanted with mouse or human hematopoietic stem cells (HSCs).22 This approach induces Wnt target gene expression via affecting the scaffolding proteins APC and Axin. However, GSK3 is involved in numerous signaling events in the cell and may lead to a mechanism-based toxicity. PF-670462 is a potent (IC50 = 7.7 ± 2.2 nM) and selective (>30-fold with respect to 42 additional kinases) inhibitor of CK1ε that phosphorylates Dvl protein in the Wnt signaling.23
There is the ongoing need for inhibitors, activators and potentiators (i.e. agents that mediate and/or enhance the effect of endogenous Wnt ligands) of Wnt signaling with novel mechanism of action. We combined our novel chemistries and structure-based approach to assemble a library of ca. 10,000 lead-like compounds able to tackle various aspects of Wnt/Fz signaling. Specific targets representing Wnt/Fz cascade include sFRP1, Dkk1, DyrK1A/B and interfaces of β-catenin with several transcription factors including Tcf-4 and Bcl-9. With this library we allow using either target-based, reporter-based or phenotypic assay(s) to test these compounds. Identified hits are expected to be tractable (i.e. feasible SAR and lead generation) and suitable as entry points into developing agents for the treatment of tumors, bone disease, Cardiovasular (CV) /metabolic, gastrointestinal (GI), and CNS/neurodegenerative disorders.
Specific targets and nodules of Fz/Wnt signaling cascade used for the library design:
Wnt/sFRP-1 interaction inhibitors. sFRP-1 family of proteins includes endogenous molecules that bind both Wnt ligands and Fz receptors. Inhibition of sFRP-1 may result in vicarious activation of Wnt signaling. In addition, WAY 316606 is a sFRP-1 inhibitor (Ki = 80 nM) that was described to increase total bone area in a murine calvarial organ culture assay at <1 nM concentration24. It was also reported to be an antiosteoporotic agent25. WAY-262611 is a Wnt pathway agonist that increases bone formation rate with EC50 of 0.63 uM in TCF-Luciferase assay. WAY-262611 exhibits good pharmacokinetic properties and a dose-dependent increase in the trabecular bone formation rate in ovariectomized rats following oral administration26. Specific attention needs to be paid to monitoring specific organ toxicity associated with a long-term perturbation of Wnt pathway including neoplasia(s),27,28 although recent data suggest otherwise29. This selection is represented by ca 1200 compounds.
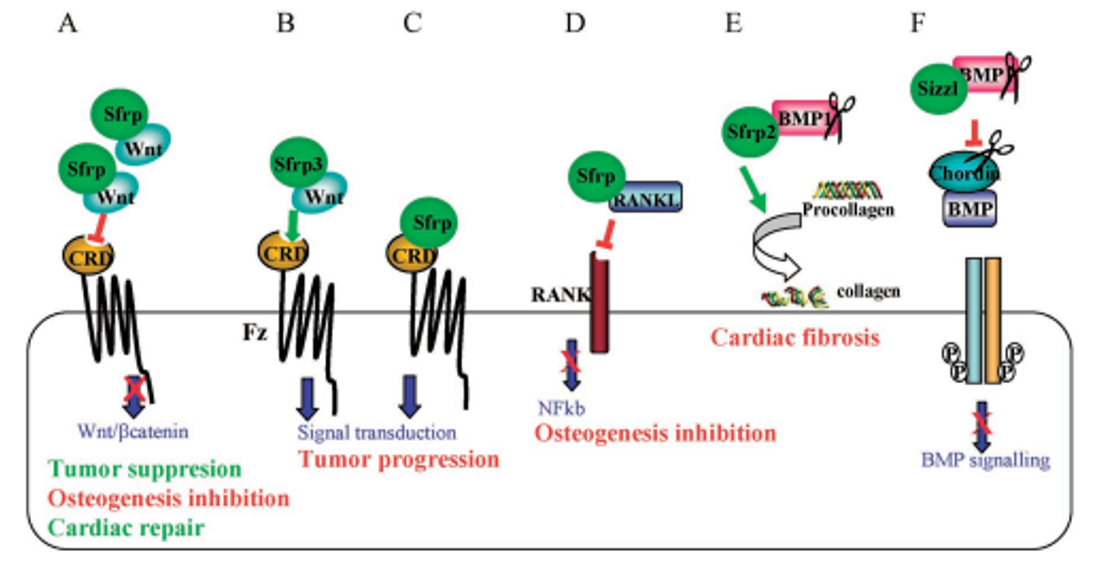
Figure 2. Multifunctional molecular interactions of sFRP1/2 and their implications in pathological events. A) sFRP1/2 sequester Wnts, thereby antagonizing Wnt/β-catenin signaling; B) Frzb (sFRP3) and Crescent favor the diffusion of Wnts, enhancing their signaling range; C) sFRP1/2 cind to Fz receptors activating intracellular signaling; D) sFRP1 binds RANKL preventing its interaction with RANK receptor; E) sFRP2 binds to and enhances the procollagen proteinase activity of BMP1/Tollpid-like metalloproteinases accelerating the processing of pro-collagen; F) Sizzled binds to and inhibits the Chordinase activity of BMP1/Tolloid-like metalloproteinase; unproteced Chordin binds and sequesters BMPs (from Tohoku J. Exp. Med. 2010, 221, 11-17).
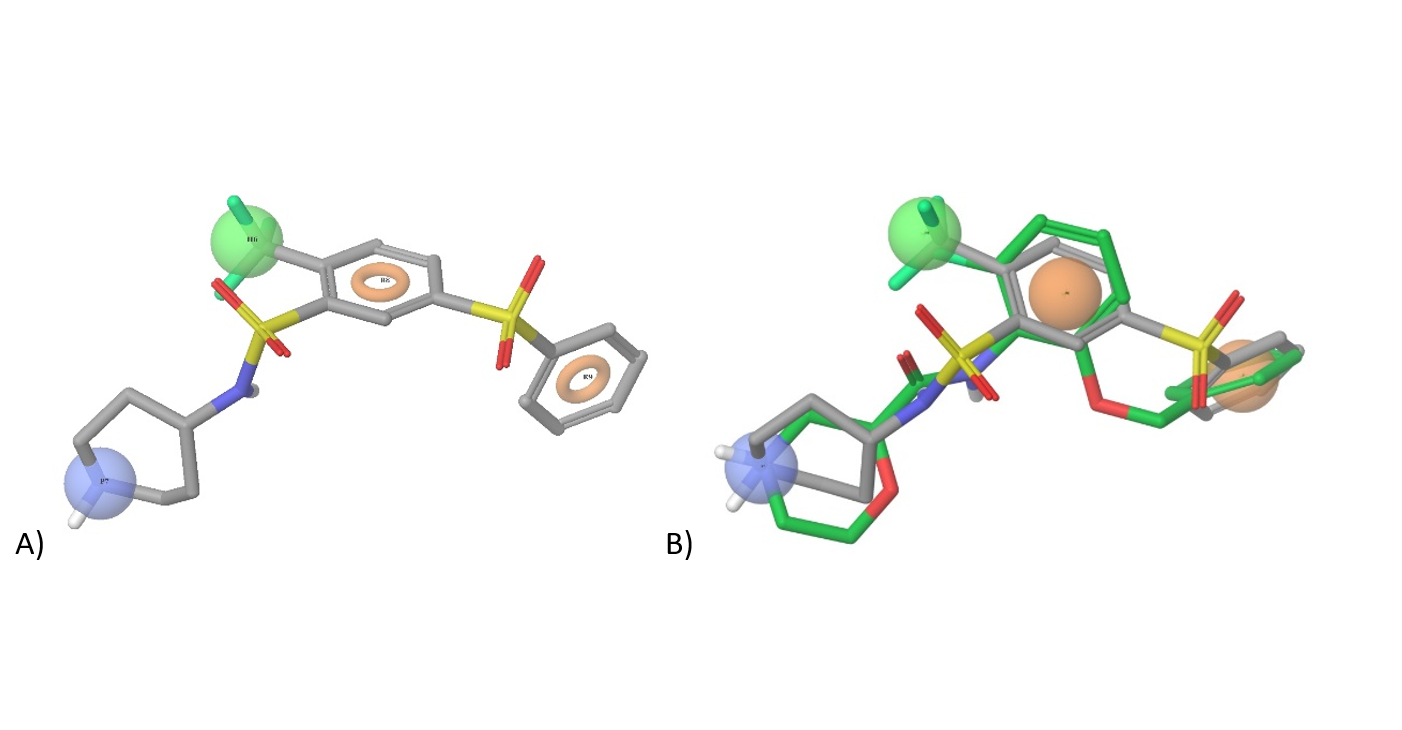
Figure 3. A) Pharmacophore model based on WAY 316606; orange – aromatic/heteroaromatic groups, green-hydrophobic pharmacophore (halogen, CF3, CHF2, Me, Et, iPr etc.), blue-positively charged moiety (+NH3Alk, +NH2Alk2, +NHAlk3 etc.); B) Superposition of WAY-316606 and our pharmacophore construct used in (sub) library design
DKK-1 Inhibitors. We used both docking and ligand-based approaches to search for potential DKK-1 binders. Specifically, the DKK1/LRP6 protein-protein interaction (PPI) interface was utilized to select matching ligands from the entire Enamine Screening Collection. The analogs of NCI8642 and other reported DKK-1/LRP6 interaction inhibitors were used for ligand-based search and pharmacophore modeling. In the docking calculation, we focused on topological and charge distribution features of the critical Dkk-1 loop that binds to LRP6. We reasoned that blocking/destabilizing this loop feature of the DKK-1/LRP6 PPI may yield modulators and/or activators of Wnt signaling while maintaining ‘normal’ Wnt-Fz-LRP6 signaling.
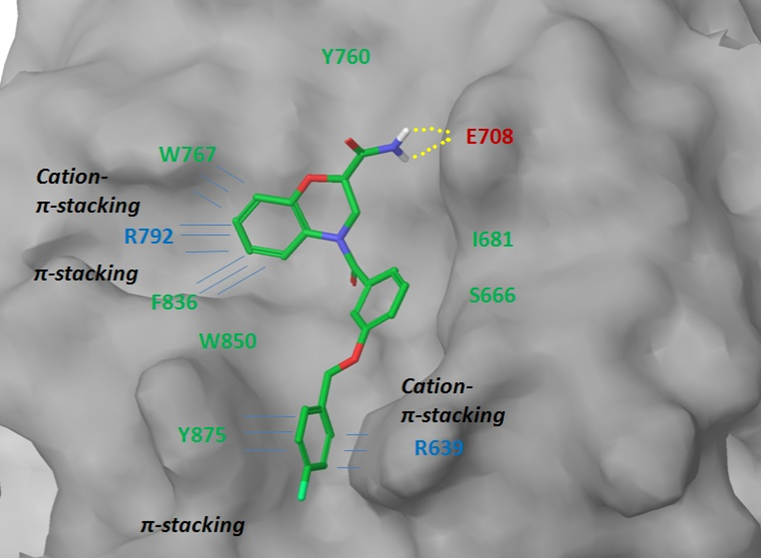
Figure 4. Binding interface between LRP6 protein and representative ligand from the focused subset.
We used known DKK-1 inhibitor WAY 262611 and its 3D pharmacophore in the ligand-based approach to identify 3k+ lead-like compounds as potential binders.
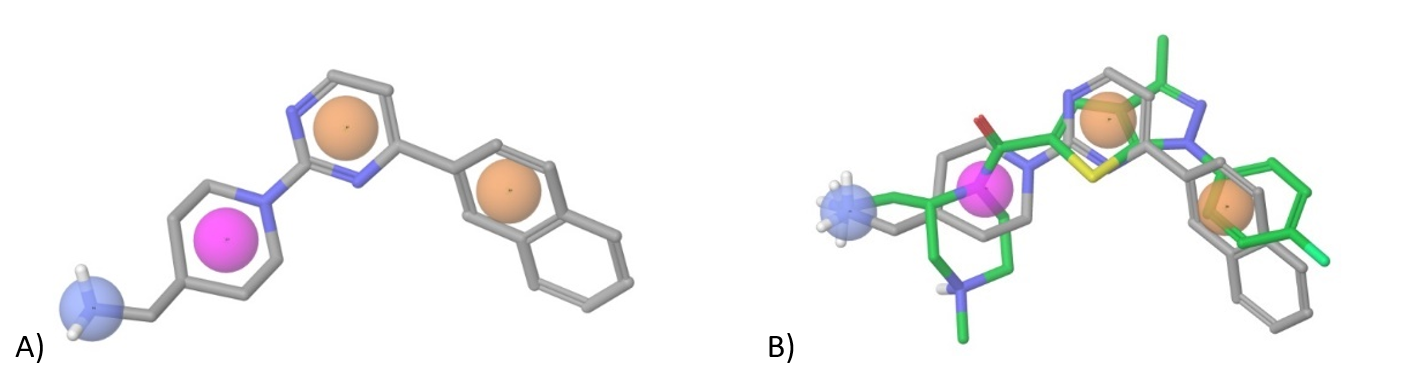
Figure 5. A) Pharmacophore model based on WAY-262611; orange – aromatic/heteroaromatic groups, blue – positively charged moiety (+NH3Alk, +NH2Alk2, +NHAlk3 etc.), magenta – aliphatic ring; B) Superposition of WAY-262611 and our pharmacophore construct used in (sub) library design.
Dvl-Axin Inhibitors. Dvl-Axin interaction is mediated by a specific PDZ-domain. Since structural information on this PPI interface is not available, we built a homology model using AlphaFold2 and numerous SBio data on PDZ domains. The resulting model was used to run docking calculation of the entire Screening Collection and identify the most promising ‘hits’. As a result, ~2500 final Dvl-biased compounds were added to the library. A representative example of putative Dvl binder is shown below.
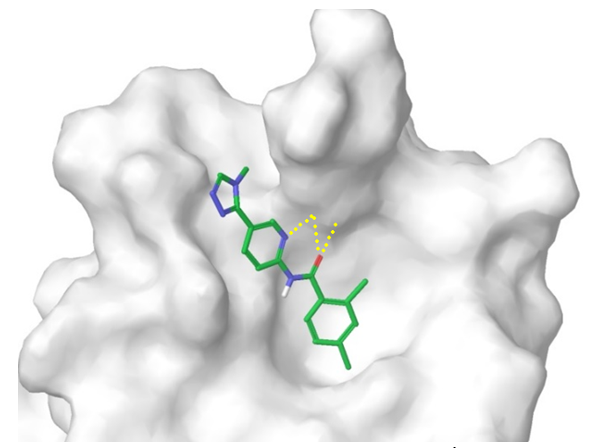
Figure 6. A representative example of Dvl/PDZ-domain binding ligand from our focused set.
β-Catenin/TCF4 Protein-Protein Interaction. To model the interaction interface between β-catenin and Tcf-4 we used reported structural data (ACS Chem. Biol. 2014, 9, 193−201) to identify critical contacts including intriguing protonated pyridine (cation) – π-interaction between docked ligand and Arg474/515 tweezers. The topology of the hydrophobic pocket and H-bond of the aromatic NH2 group with protein’s Lys508 residue allowed construction of a respective pharmacophore model (Figure 7). After docking calculation and scoring cut off, a focused selection of ca. 1800 small molecules was assembled into β-Catenin/TCF4 sublibrary.
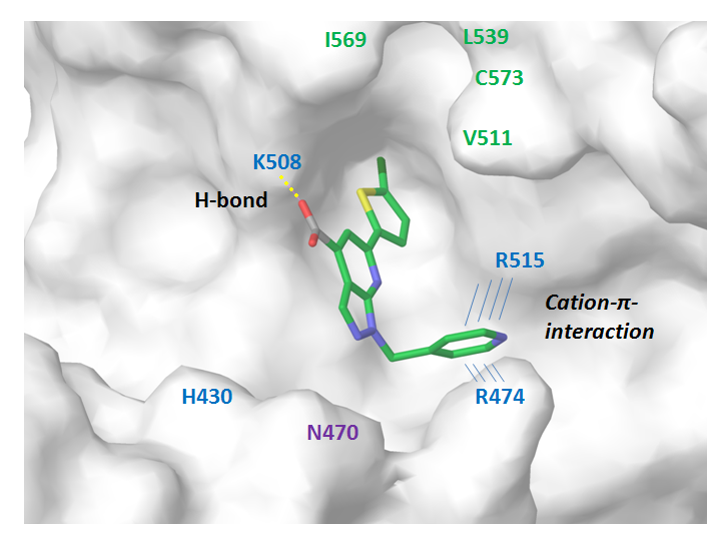
Figure 7. Binding interface between β-catenin and representative ligand from the focused subset.
β-Catenin/Bcl9 Protein-Protein Interaction Interface. We focused on the available structural data of the reported helix-helix interaction between the two biological molecules. Specifically, we investigated the key H-bonds between H358, R359 (Bcl9) and D162, D164 (β-catenin) (Figure 8). Following docking and binding poses prioritization, we picked molecules that secured hydrogen bonding with D162/D164 and exhibited a significant π-stacking/hydrophobic interaction area with β-catenin to result in the 1779-compound library.
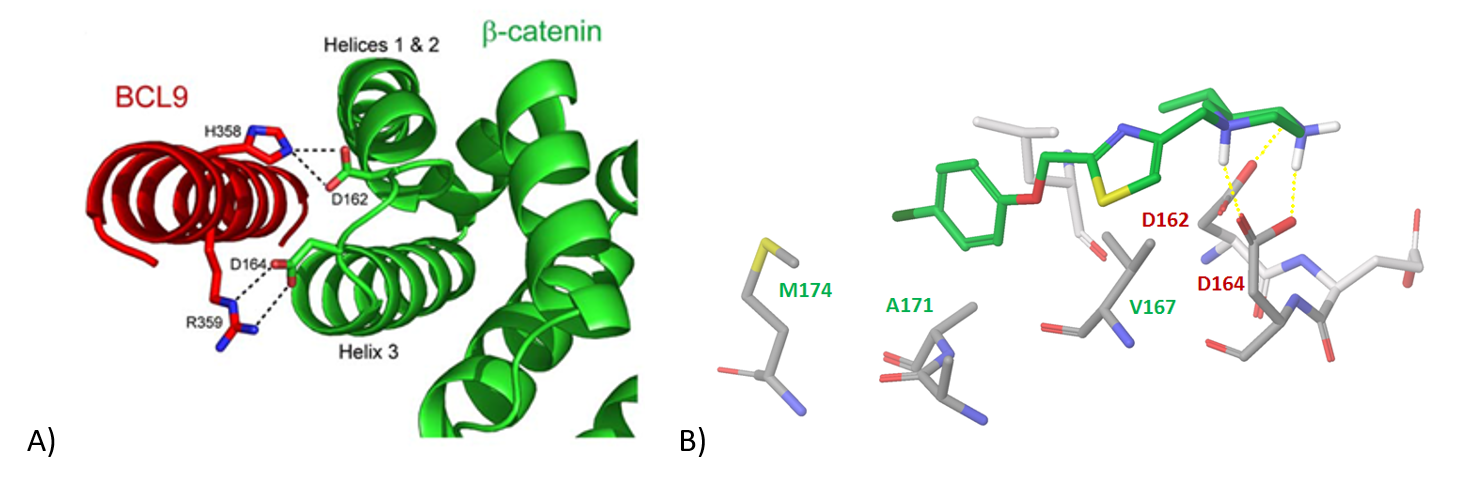
Figure 8. A) β-catenin/BCL9 helix-helix interaction interface featuring key hydrogen bonds; B) binding of β-catenin and a representative ligand from the focused subset.
Bcl9/PYGO/Histone 3 Interaction Interface. The other option to block Wnt/Fz signaling is disruption of PYGO/Histone 3 interaction (Figure 9A). Notably, it has been shown that small molecules could bind to the PHD domain of PYGO that engages the methylated Histone 3 residue, which leads to disruption of β-catenin/Bcl9 interaction. After docking calculation and scoring prioritization, 318 small molecules were selected as potential PPI inhibitors of this complex. The representative example of a ligand mediating multiple hydrophilic interactions, p-stacking with Phe366 and hydrogen bonds, is shown in Figure 9B.
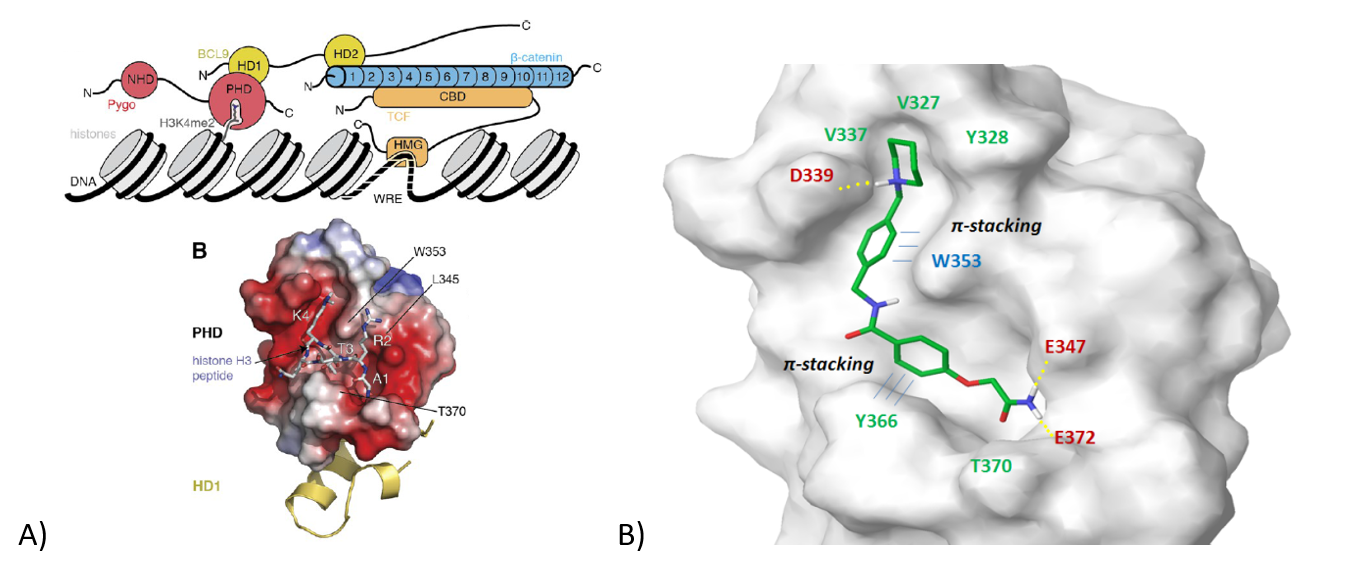
Figure 9. A) Interaction interface of BCL9/PYGO/Histone 3; B) Representative molecule bound to the key amino acids of H3K4me2.
References
-
Interaction of Wnt and Activin in Dorsal Mesoderm Induction in Xenopus
Sokol, S. Y.; Melton, D. A. Dev. Biol.1992, 154, 348-355. doi:10.1016/0012-1606(92)90054-L -
A role for maternal β-catenin in early mesoderm induction in Xenopus
Schohl, A.; Fagotto, F. EMBO J.2003, 22, 3303-3313. doi:10.1093/emboj/cdg303 -
Neural induction in Xenopus requires inhibition of Wnt/β-catenin signaling
Heeg-Truesdell, E.; LaBonne, C. Dev. Biol.2006, 298, 71-86. doi:10.1016/j.ydbio.2006.06.004 -
The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells
Hirabayashi, Y.; Itoh, Y.; Tabata, H.; Nakajima, K.; Akiyama, T.; Masuyama, N.; Gotoh, Y. Development2004, 131, 2791-2801. doi:10.1242/dev.01130 -
WNT Signaling molecules act in axis formation in the diploblastic metazoan Hydra
Hobmayer, B.; Rentzsch, F.; Kuhn, K.; Happel, C. M.; Cramer von Laue, C.; Snyder, P.; Rothbacher, U.; Holstein, T. W. Nature2000, 407, 186-189. doi:10.1038/35025063 -
Wnt/β-catenin signaling and body plan formation in mouse embryos
Marikawa, Y. Semin. Cell Dev. Biol.2006, 17, 175-184. doi:10.1016/j.semcdb.2006.02.009 -
Wnt signaling in disease and in development
Nusse, R. Cell Res.2005, 15, 28-32. doi:10.1038/sj.cr.7290260 -
A role for Wnt signaling in self-renewal of haematopoietic stem cells
Reya, T.; Duncan, A. W.; Ailles, L.; Domen, J.; Scherer, D. C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I. L. Nature2003, 423, 409-414. doi:10.1038/nature01593 -
Wnt/β-catenin Is Essential for Intestinal Homeostasis and Maintenance of Intestinal Stem Cells
Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Mol. Cell. Biol.2007, 27, 7551-7559. doi:10.1128/MCB.00104-07 -
Wnt signaling function in Alzheimer’s disease
De Ferrari, G. V.; Inestrosa, N. C. Brain Res. Rev.2000, 33, 1-12. doi:10.1016/S0165-0173(00)00018-3 -
Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration
Li, H.; Richardson, W. D. Nat. Neurosci.2009, 12, 815-817. doi:10.1038/nn.2320 -
Wnt signaling in polycystic kidney disease
Benzing, T.; Simons, M.; Walz, G. J. Am. Soc. Nephrol.2007, 18, 1389-1398. doi:10.1681/ASN.2007020158 -
Islet Specific Wnt Activation in Human Type 2 Diabetes
Lee, S. H.; Demeterco, C.; Geron, I.; Abrahamsson, A.; Levine, F.; Itkin-Ansari, P. Exp. Diabetes Res.2008, 13. doi:10.1155/2008/728763 -
Wnt signaling in rheumatoid arthritis
Sen, M. Rheumatology2005, 44, 708-713. doi:10.1093/rheumatology/keh588 -
Wnt signaling in lung cancer
Mazieres, J.; He, B.; You, L.; Xu, Z.; Jablons, D. M. Cancer Lett.2005, 222, 1-10. doi:10.1016/j.canlet.2004.09.041 -
Wnt signaling in breast cancer: have we come full circle?
Brown, A. M. C. Breast Cancer Res.2001, 3, 351-355. doi:10.1186/bcr313 -
WNT1-induced secreted protein (WISP1), a novel regulator of bone turnover and Wnt signaling
Maeda, A.; et al. J. Biol. Chem.2015, 290, 14004-14018. doi:10.1074/jbc.M115.642306 -
Deficient Wnt signaling triggers striatal synaptic degeneration and impaired motor behavior in adult mice
Galli, S.; et al. Nat. Commun.2015, 5, 4992. doi:10.1038/ncomms5992 -
Directed cardiomyogenesis of human pluripotent stem cells by modulating Wnt/β-catenin and BMP signaling with small molecules
Aguilar, J. S.; et al. Biochem. J.2015, 469, 235-241. doi:10.1042/BJ20150274 -
Identification of 2-aminopyrimidine derivatives as inhibitors of canonical Wnt signaling pathway
Del Bello, F.; et al. Bioorg. Med. Chem.2015, 23, 5725-5733. doi:10.1016/j.bmc.2015.06.052 -
Wnt/β-catenin signaling and disease
Clevers, H.; Nusse, R. Cell2012, 149, 1192-1206. doi:10.1016/j.cell.2012.05.012 -
Regulation of Wnt signaling during adipogenesis
Bennett, C. N.; et al. J. Biol. Chem.2002, 277, 30998-1004. doi:10.1074/jbc.M204527200 -
Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases
Perez, D. I.; Gil, C.; Martinez, A. Med. Res. Rev.2011, 31, 924-954. doi:10.1002/med.20202 -
A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation
Bodine, P. V. N.; et al. Bone2009, 44, 1063-1068. doi:10.1016/j.bone.2009.02.011
