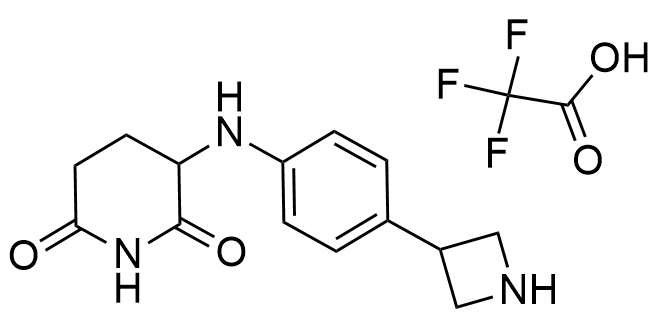
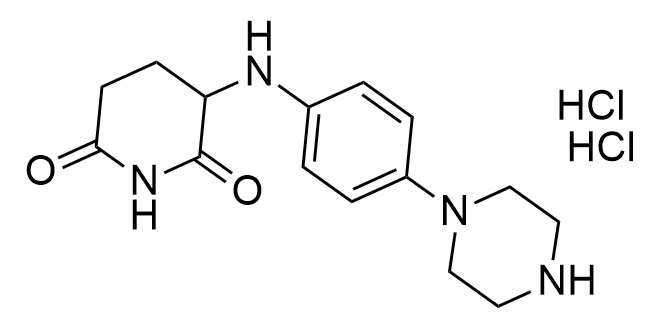
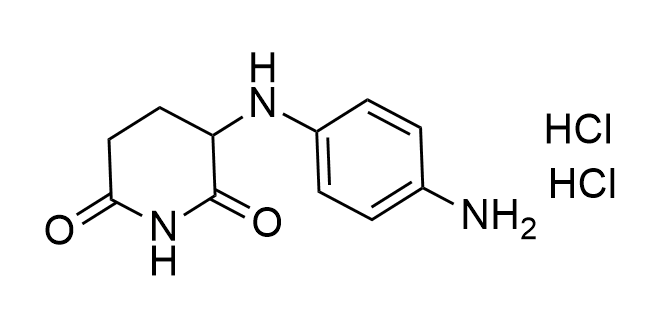
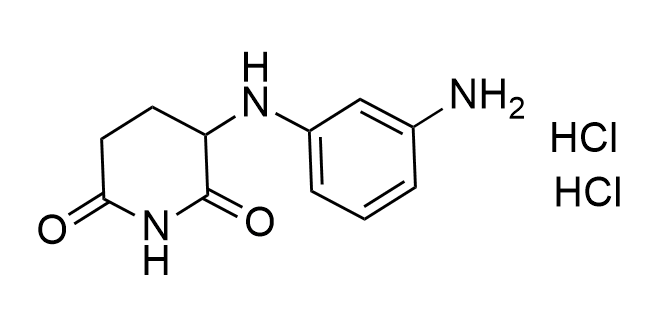
Developed to expand the structural diversity of Molecular Glues
640 compounds
The Phenyl Amino Glutarimide (PAG) scaffold is an alternative binder to the CRBN E3 ligase. This type of binder retains the same binding capability to the protein and a similar physicochemical profile, while offering a 16% reduction in molecular weight. This makes the PAG scaffold an attractive alternative candidate for the design of various Molecular Glues.
Our library is 80% designed around published and validated Phenyl Amino Glutarimide (PAG) CRBN E3 binders with high variability in exit vectors. The remaining 20% of the library is built around various heteroaromatic amino glutarimides.
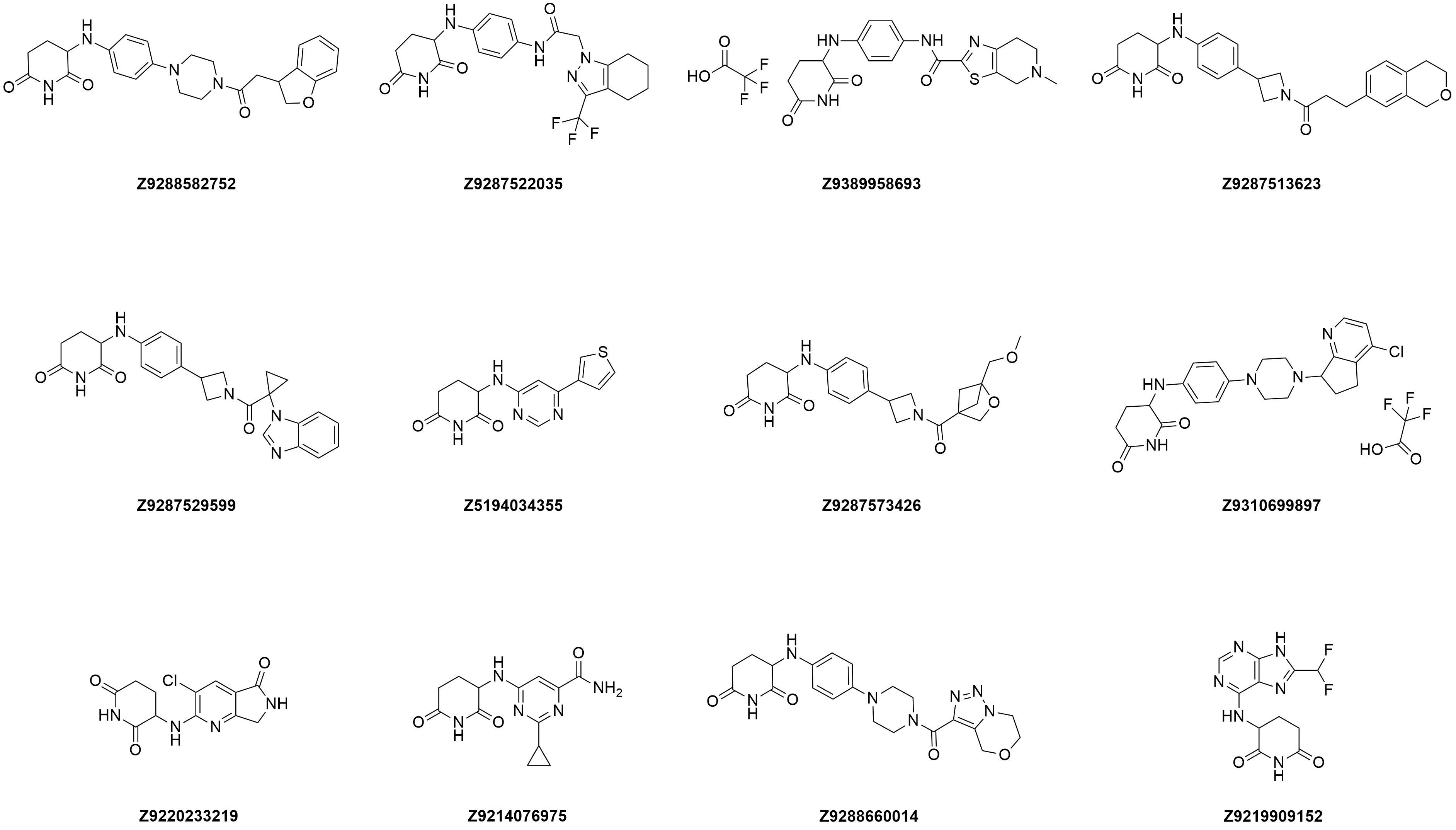
Representative examples of building blocks for library design
Examples of the molecules in the library
Download SD file
Typical Formats
Catalog No.
PAG-640-0-Z-20
Compounds
640
1 plate
Amount
≤ 300 nL of 20 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
PAG-640-10-Y-20
Compounds
640
2 plates
Amount
10 µL of 20 mM DMSO solutions
Plates and formats
384-well Echo plates, Labcyte #LP-0200, 320 compounds per plate, first two and last two columns empty
Price
Catalog No.
PAG-640-50-X-20
Compounds
640
8 plates
Amount
50 µL of 20 mM DMSO solutions
Plates and formats
96-well plates, 80 compounds per plate, first and last columns empty; Greiner #781270
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Selected publication
-
Design and Synthesis of Novel Cereblon Binders for Use in Targeted Protein Degradation
.
J. Med. Chem. 2023, 66, 23, 16388–16409. DOI: 10.1021/acs.jmedchem.3c01848 -
Evaluation of Cereblon-Directing Warheads for the Development of Orally Bioavailable PROTACs.
J. Med. Chem. 2025, 68, 3, 3591–3611. DOI: 10.1021/acs.jmedchem.4c02709
Support
We offer comprehensive support in developing your hit compounds. Naturally such programs are realised most efficiently when biological actives originate from our screening collection. However, even if the hit compounds are from the collections of other vendors lead identification and optimization projects can proceed most productively in our hands. Sometimes for this we only need to synthesize first examples of the given chemical series and validate synthesis route.