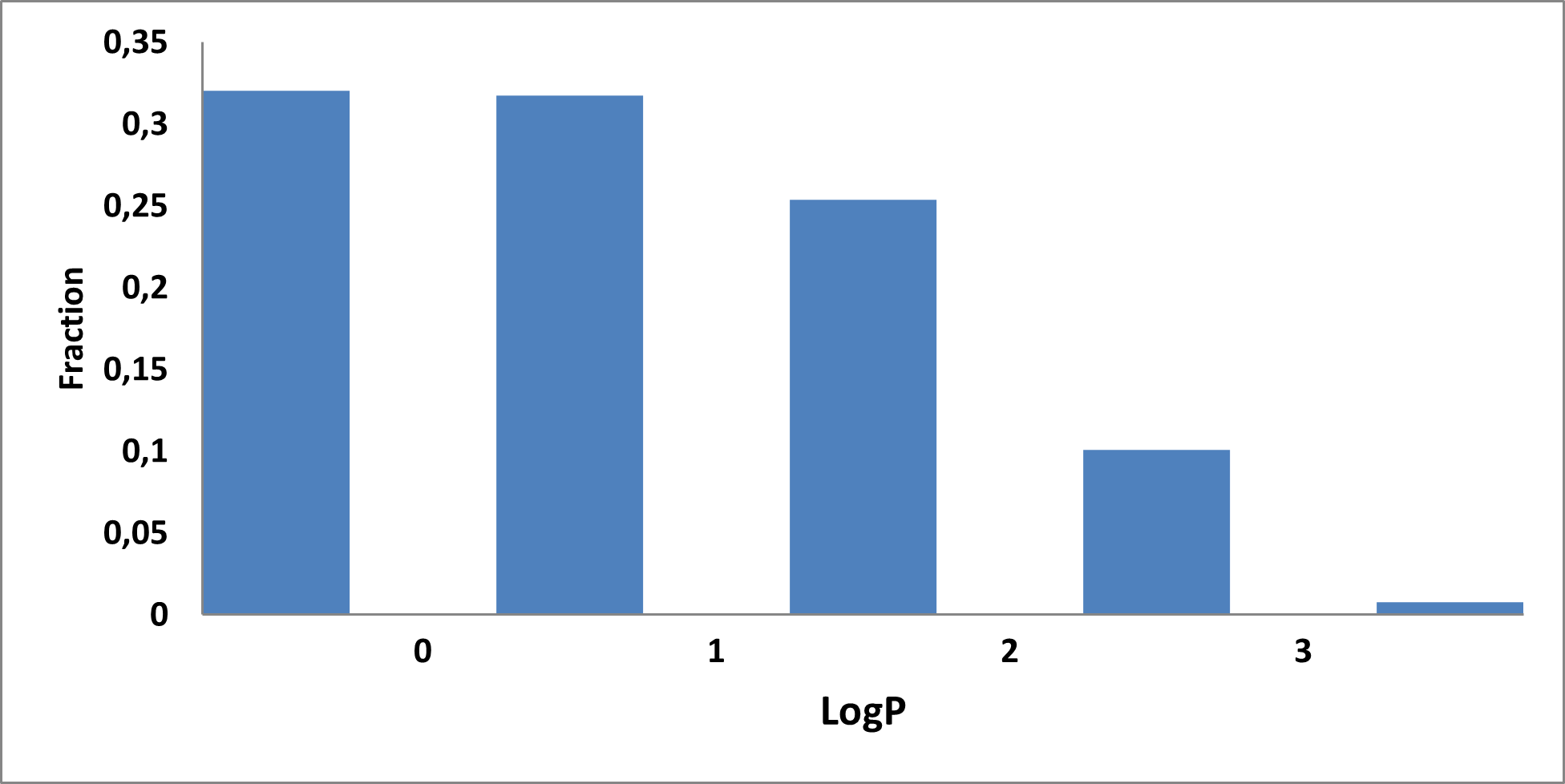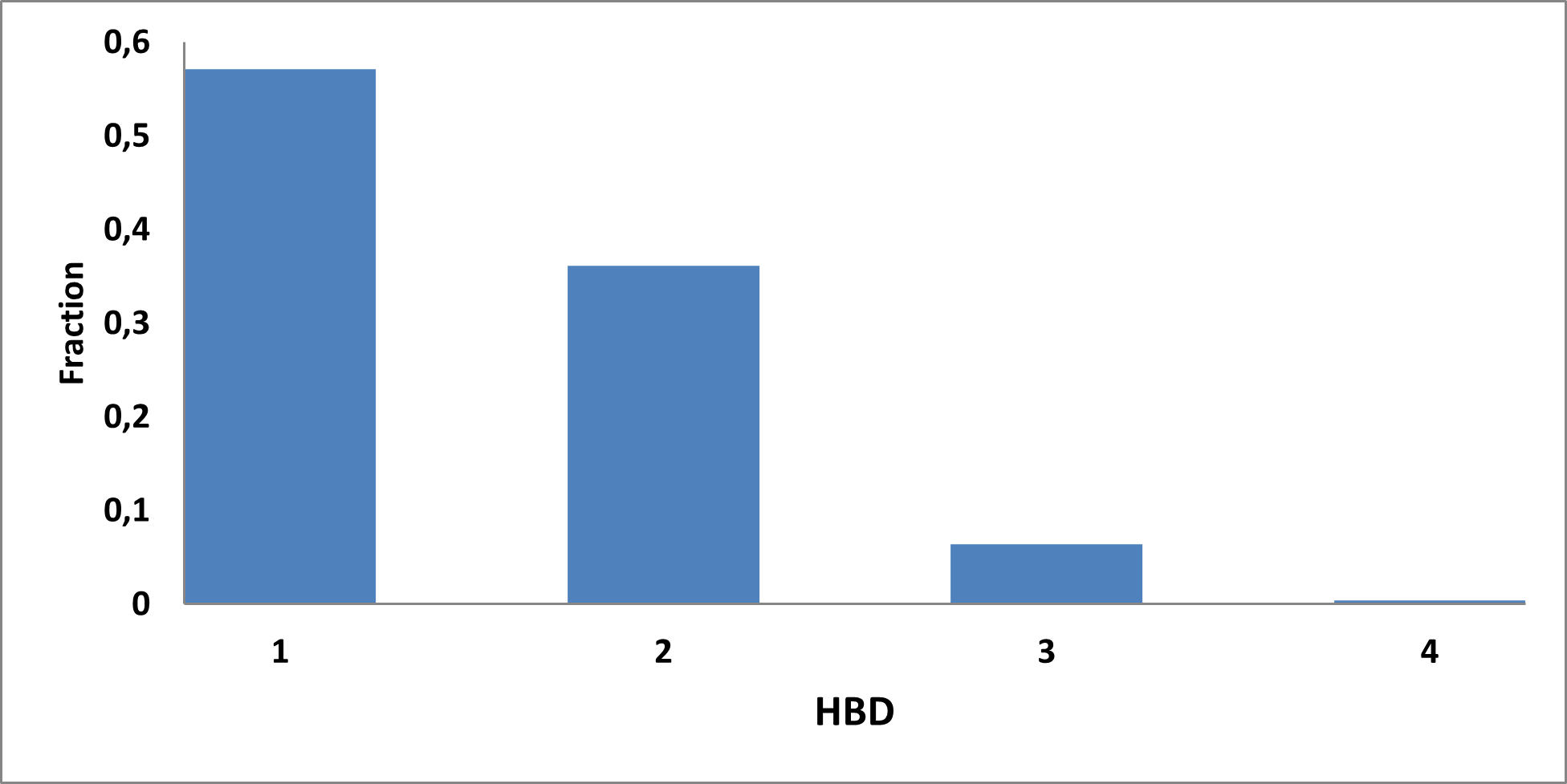The most medchem reliable source of carboxylic acids replacement
1 280 compounds
Together with The Institute of Cancer Research, London, we have developed a powerful FBDD tool for searching carboxylic acid bioisostere (CAB) fragment hits. This approach provides an alternative to the conventional replacement of the carboxylic acid moiety during lead optimisation. While the -COOH group is present in several approved drugs and significantly contributes to target binding, carboxylic acids can limit cell permeability and become a tox liability. Even though many carboxylic acid bioisosteres have been reported, the outcome of any bioisosteric replacement cannot be readily predicted. This collection of CAB-frags provides a screening tool kit to identify carboxylic acid surrogates at the very outset of a drug discovery campaign to find hits that form critical binding interactions to a target of interest that may translate to a larger lead molecule.
Typical Formats
Catalog No.
CAB-1280-10-Y-100
Compounds
1 280
4 plates
Amount
10 µL of 100 mM DMSO solutions
Plates and formats
384-well plates Greiner Cat. No 781280, first two and last two columns empty, 320 compounds per plate
Price
Catalog No.
CAB-1280-25-Y-100
Compounds
1 280
4 plates
Amount
25 µL of 100 mM solutions in DMSO
Plates and formats
384-well plates Greiner Cat. No 781280, first two and last two columns empty, 320 compounds per plate
Price
Download SD file
Library code: CAB-1280
Version: 11 November 2024
1 280 compounds
at 100 mM in DMSO
Key features
- Covers all reliable CAB replacements
- Experimentally confirmed solubility in PBS buffer at 1 mM, in DMSO at 100 mM
- High diversity within each bioisosteric type
- No reactive or unstable compounds, pains free
- Analogs from stock and REAL Space available for each fragment
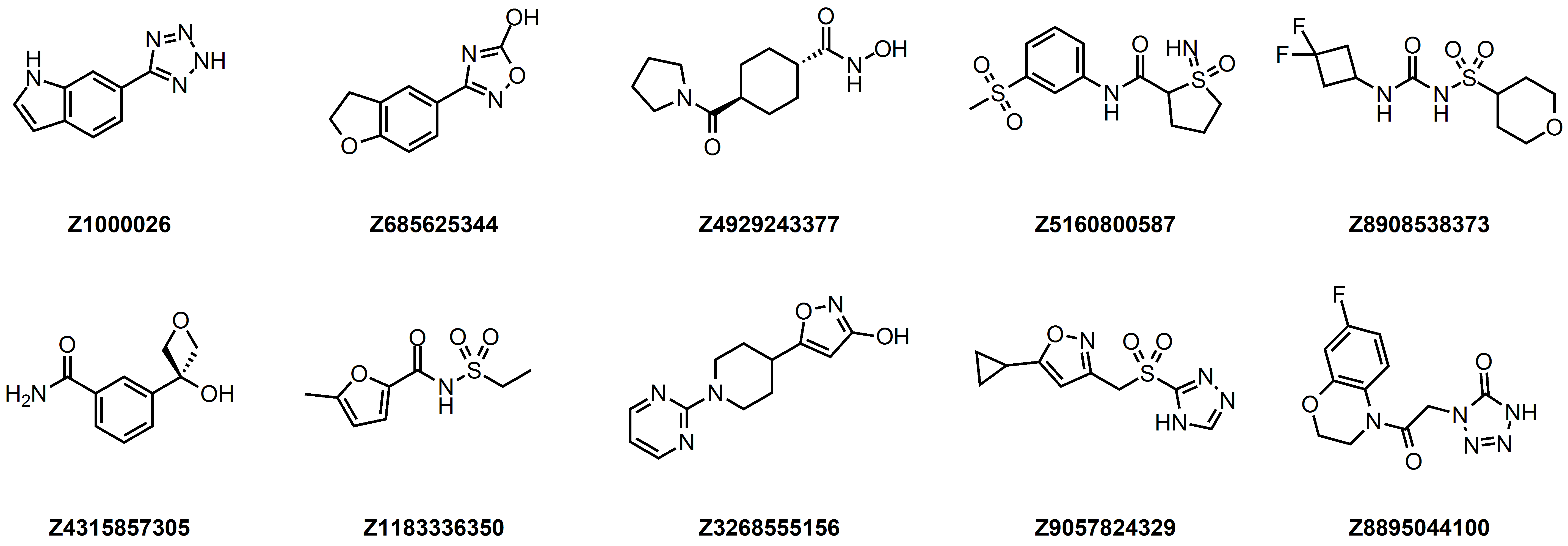
Examples of compounds in the library
Library design
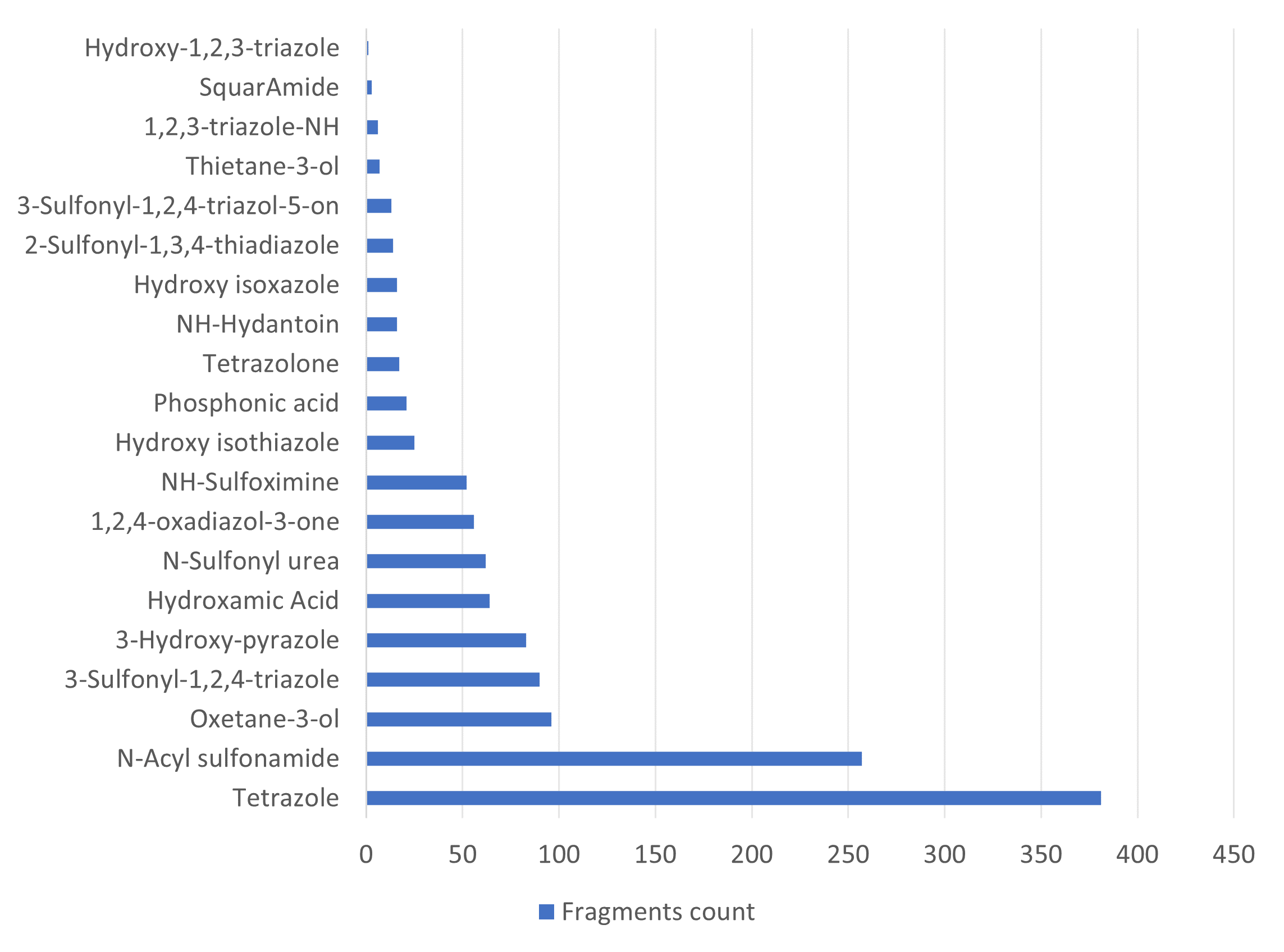
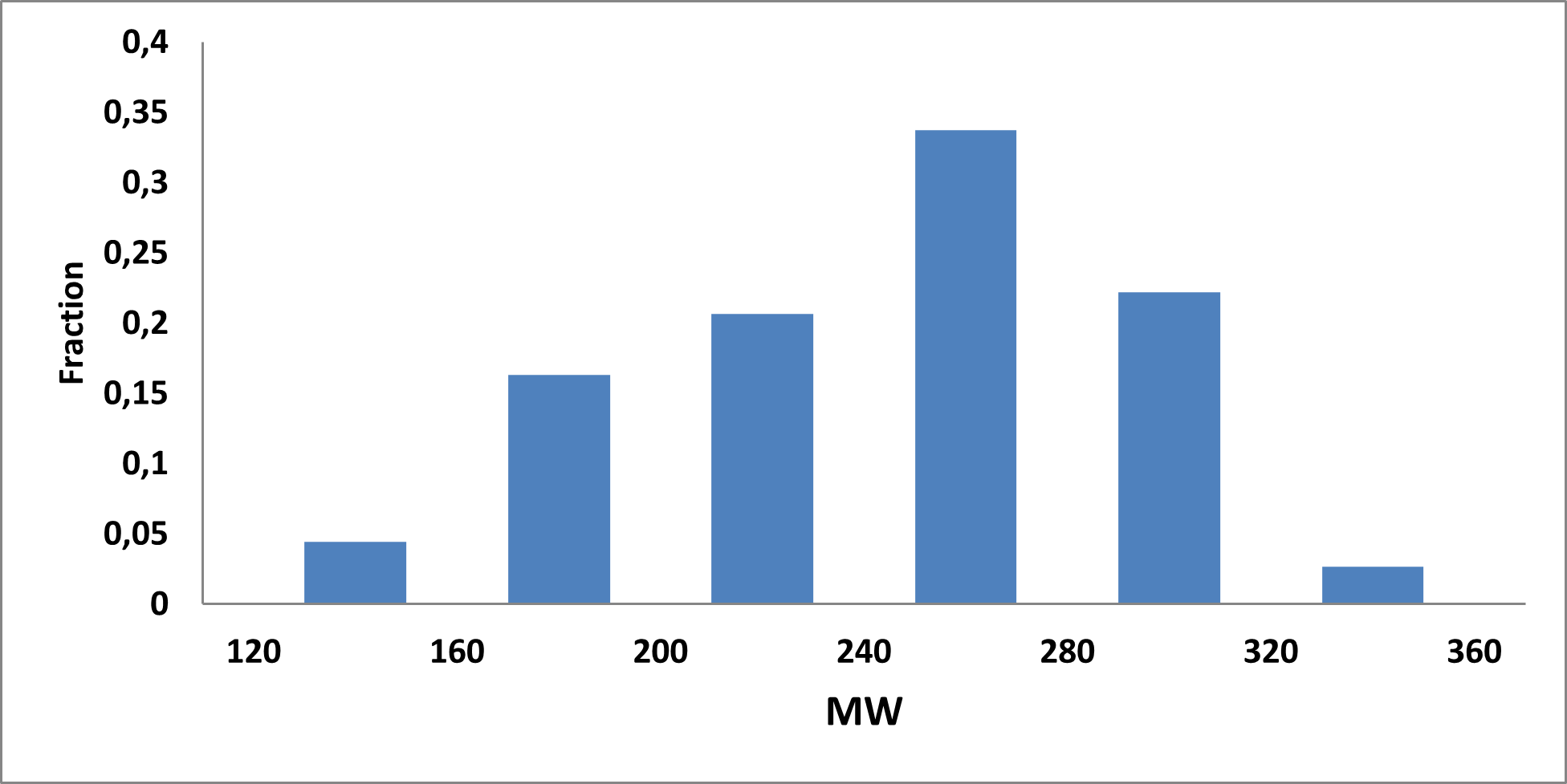
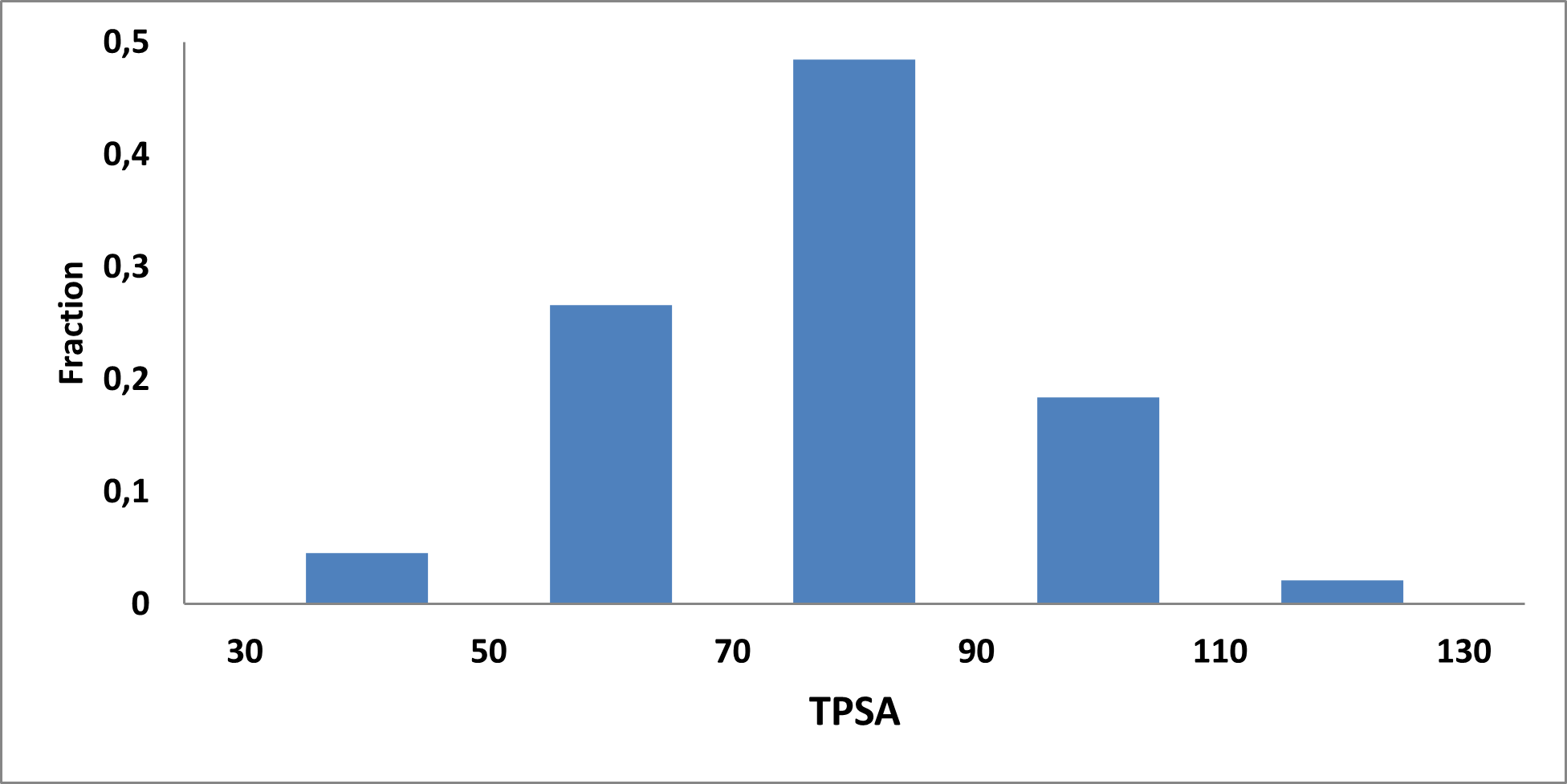
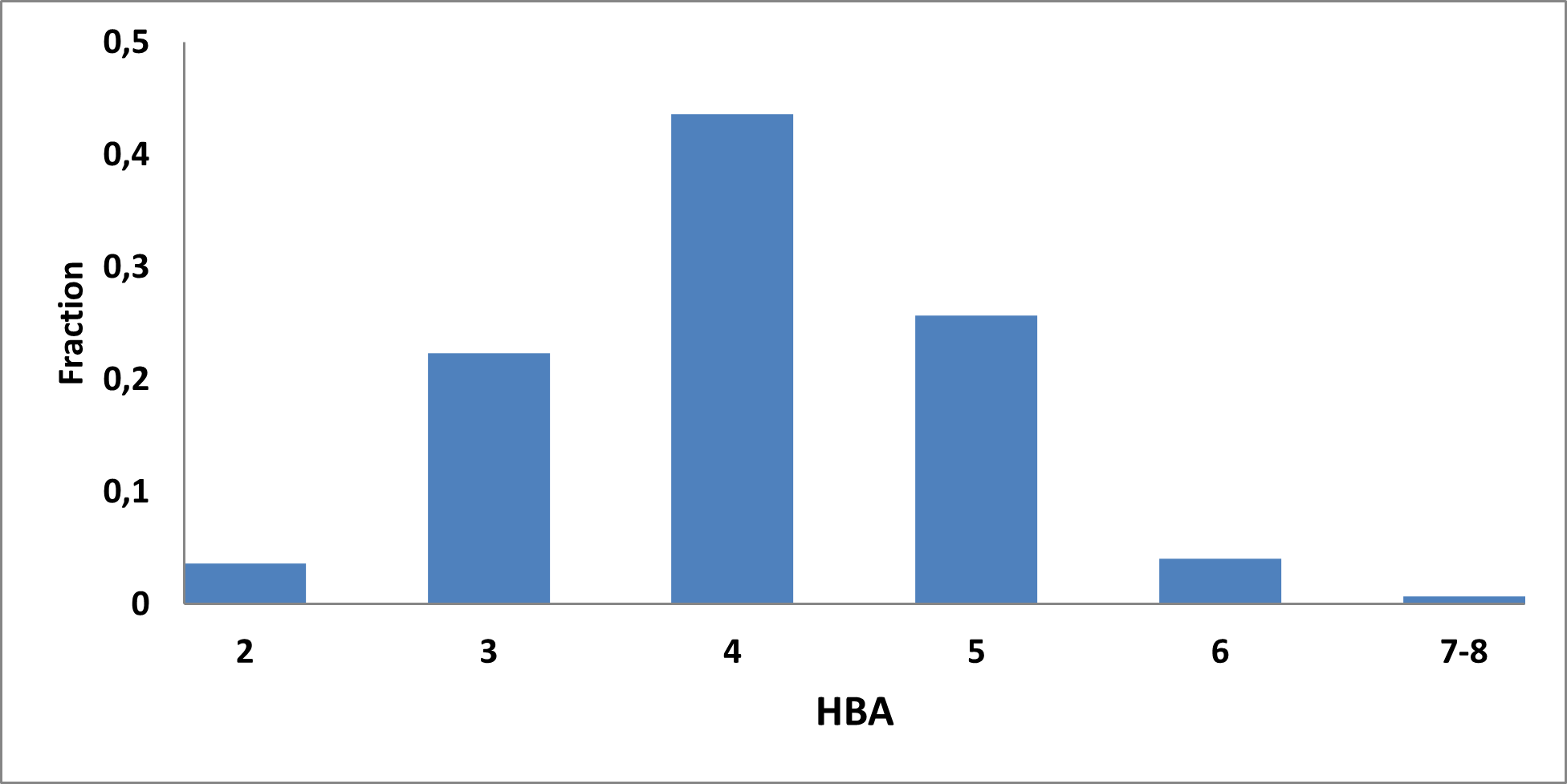
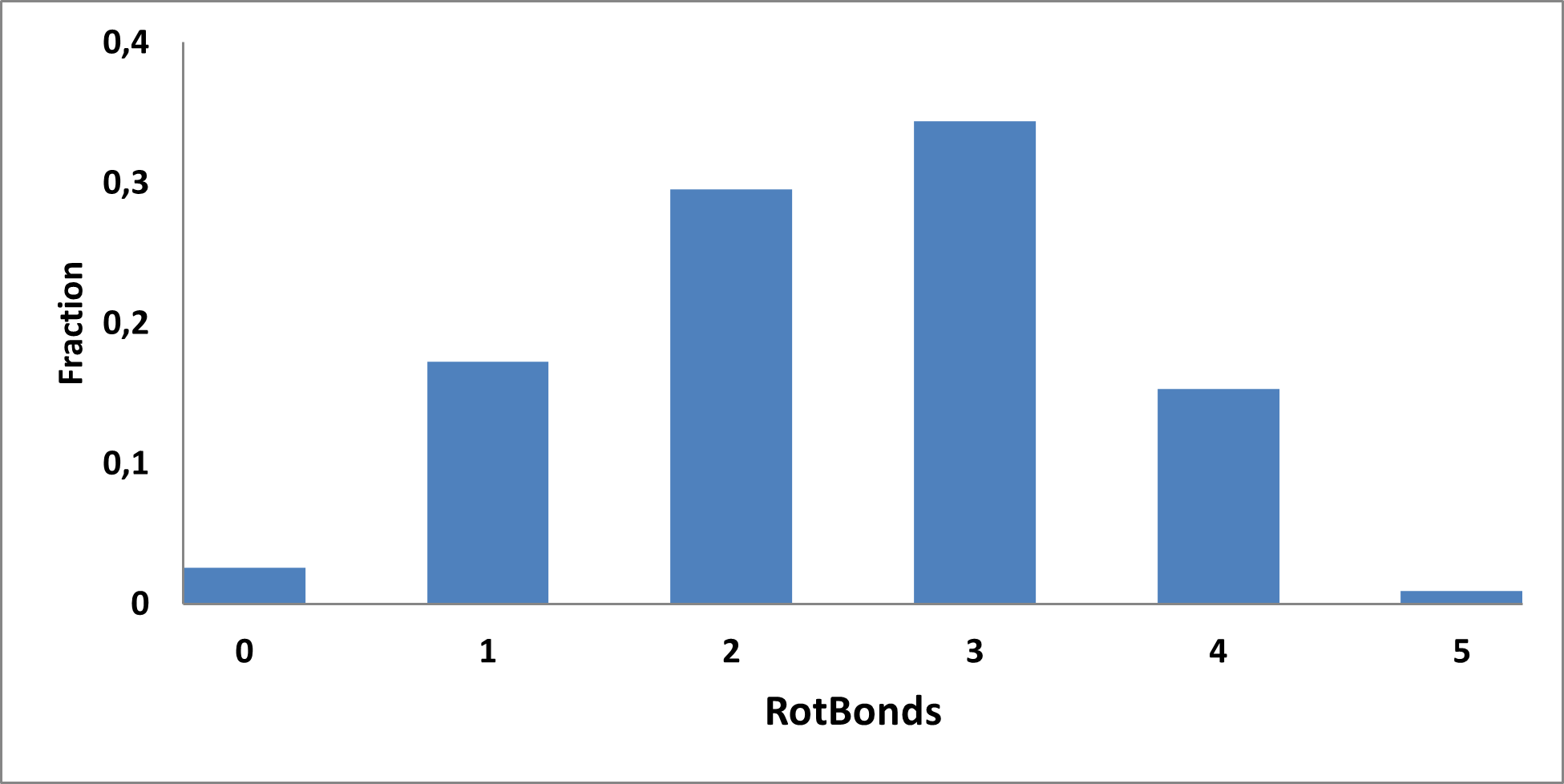
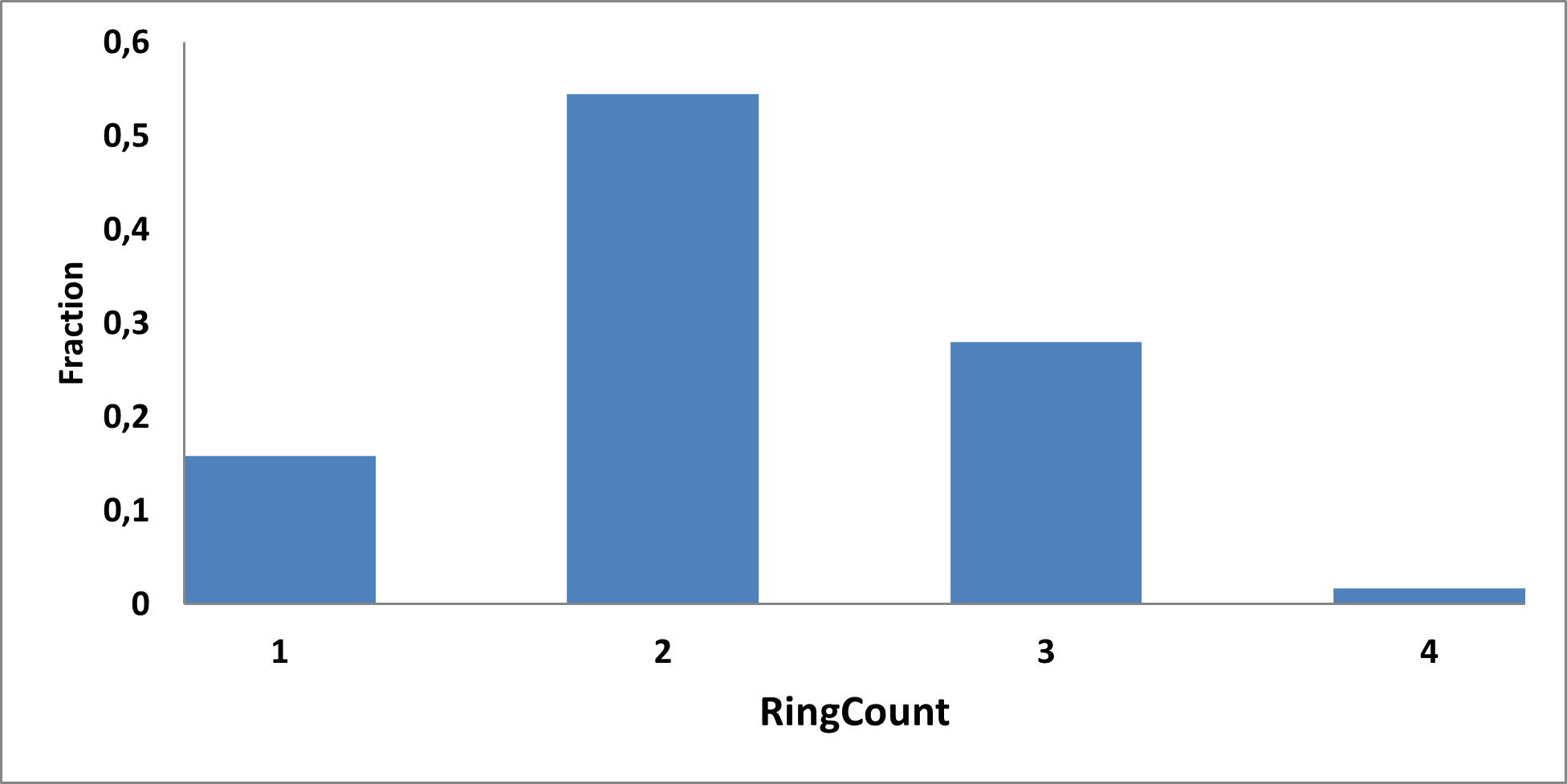
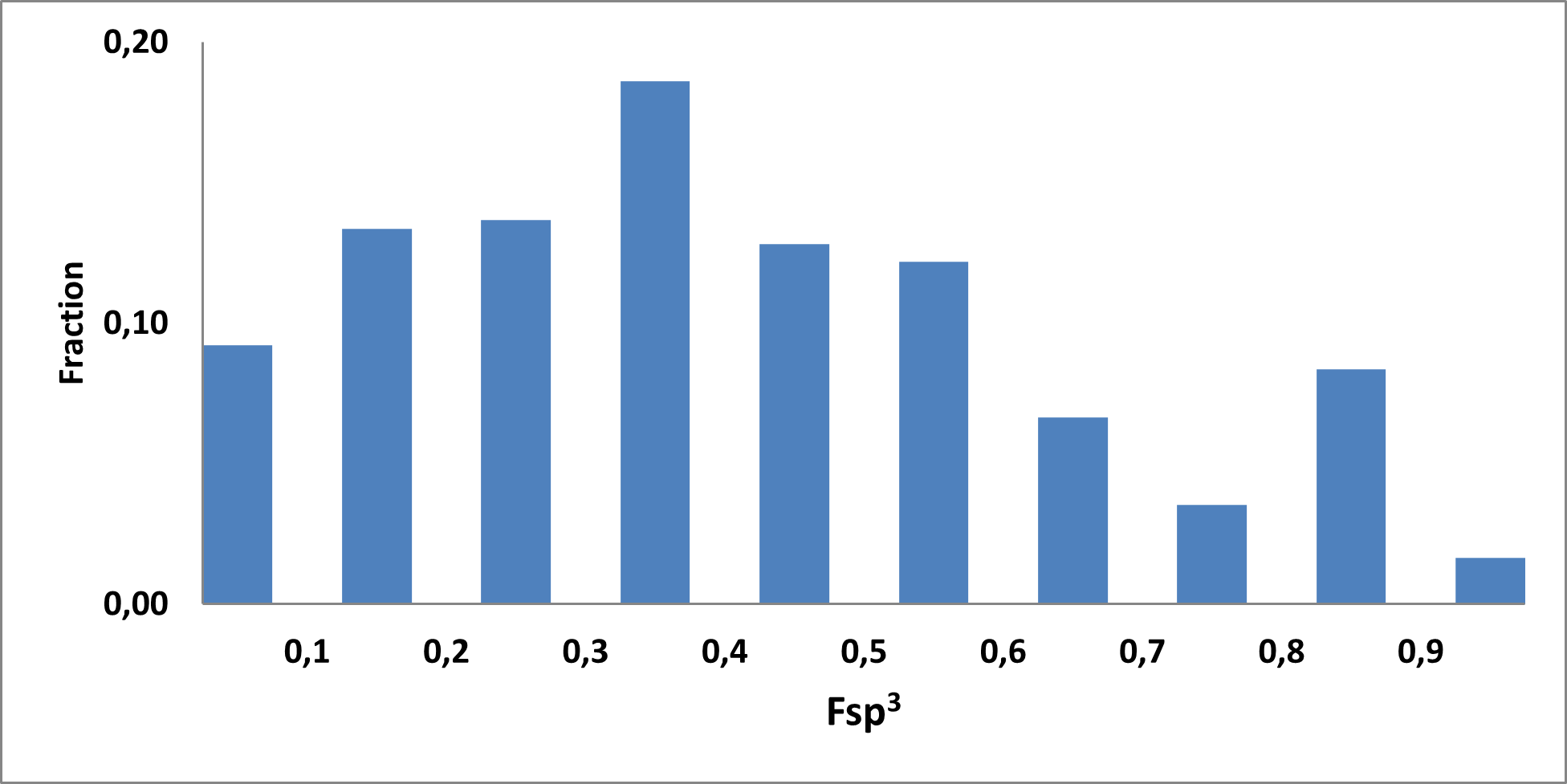
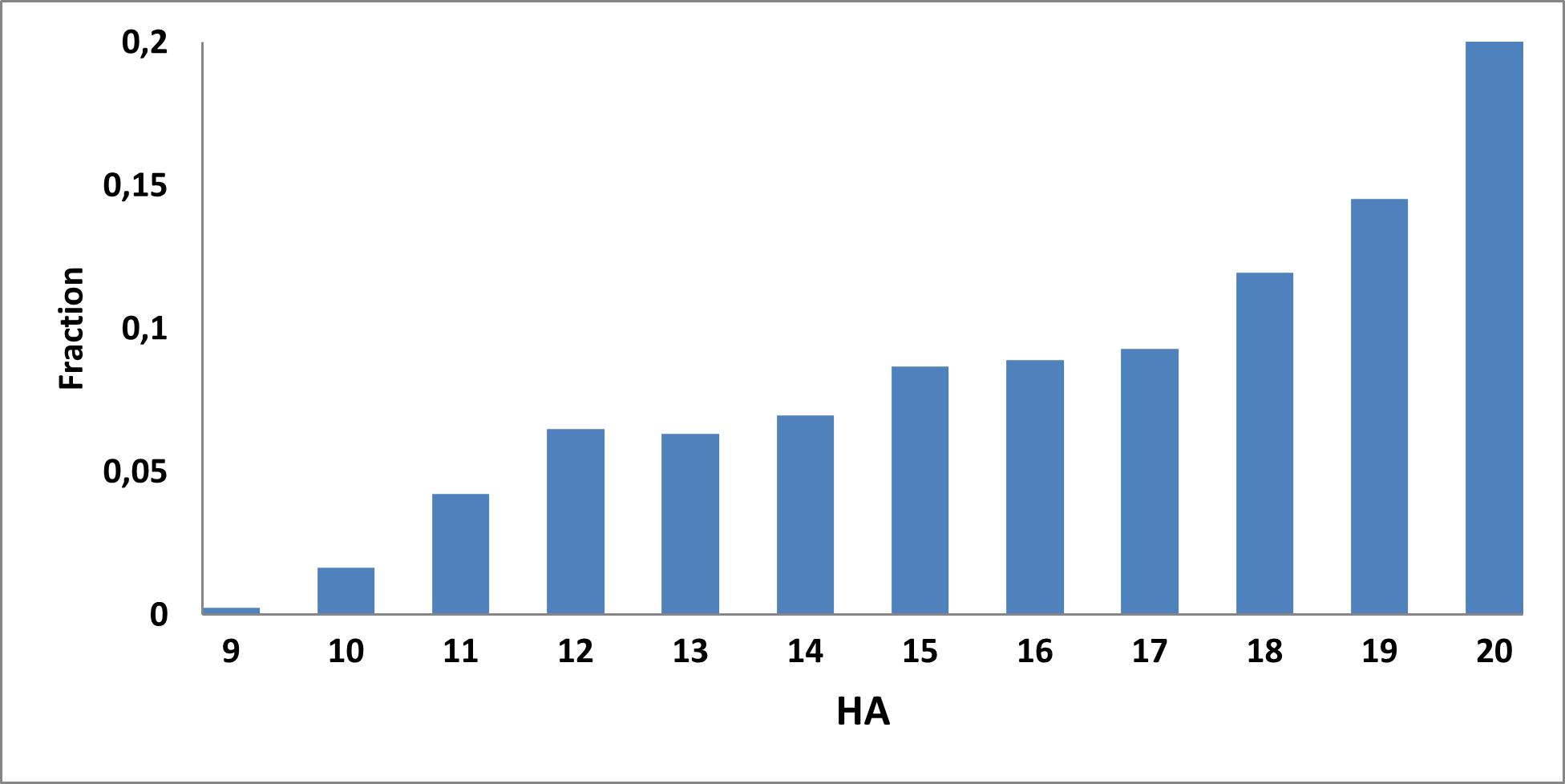
Analysis of the existing literature combined with internal experience at the ICR allowed us to choose more than thirty CAB substructure types. We identified over 40,000 compounds containing CAB moieties in our stock. Some underrepresented classes were identified, and new compounds were synthesized to fill these gaps. Compounds containing carboxylic acid groups or highly reactive groups were filtered out. A slightly relaxed version of the Rule of Three (Ro3) was applied along with diversity selection to comply with the standard practices of fragment-based library design while allowing some flexibility to enhance library size and diversity. We further extended the selection to compounds whose properties are in line with those of functionally diverse fragments that perform best in fragment screens (Deane et al., J. Med. Chem. 2022, 65, 11404−11413). The final selection contains only compounds that passed nephelometry-based solubility threshold studies (solubility in DMSO at 100mM and 1mM in PBS at pH 7.4). The library composition was visually checked by Enamine and ICR experts to remove trivial and MedChem unfriendly compounds. The distribution of CAB classes took into account both medicinal chemistry attractiveness and synthetic tractability, the latter being crucial for the hit follow-up stage. Tetrazoles and N-acyl sulfonamides are the most abundant classes in the library followed by 1,2,4-oxadiazoles, 3-sulfonyl-1,2,4-triazoles, hydroxamic acids, oxetane-3-ols, 3-hydroxypyrazoles and others with 20 CAB classes in total.