Designed for discovery of mild electrophilic inhibitors of the largest enzyme class
12 160 compounds
Serine hydrolases are important class of enzymes which include lipases, esterases, thioesterases, amidases, peptidases and proteases. They belong to one of the largest and most diverse classes of enzymes found in nature and represent ~1% of all proteins in mammalians playing vital roles in such pathophysiological processes as blood clotting, digestion, nervous system signalling, inflammation and cancer.
Despite the fact that pharmaceutical industry avoid developing therapeutics that form covalent bonds with protein targets, several commercialized serine hydrolase inhibitors as well as promising drug-candidates contain electrophilic chemical groups that interact covalently with Serine residue in the active site of their targets.
The library of mild electrophilic compounds has been plated for most convenient and quick access. Using our Serine hydrolase focused library you receive multiple benefits, allowing you to save on time and costs in hit expansion and optimization:
- Hit confirmation support – resupply of dry samples after QC check, samples repurification or resynthesis upon request.
- Analogs from dry stock of over 4.7M compounds or REAL Space of 78.1B make-on-demand molecules.
- Straightforward & low-cost synthesis of follow-up libraries through our REAL Database technology
You have also an option to screen the library directly at Enamine. In this case we will be happy to offer discount on library cost depending on the collaboration scope.
Typical Formats
Covalent Serine Hydrolase Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
CSHL-12-0-Z-10
Compounds
12 160
10 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
CSHL-12-10-Y-10
Compounds
12 160
38 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
CSHL-12-50-Y-10
Compounds
12 160
38 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, first and last two columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
CSHL-12-10-Y-10 screening library 12 160 cmpds, hit resupply, analogs from 4.7M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD files
Library code: CSHL-12160
Version: 10 January 2022
12 160 compounds
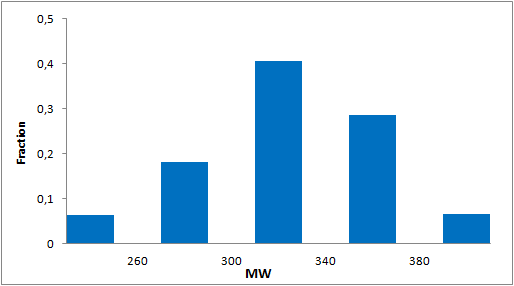
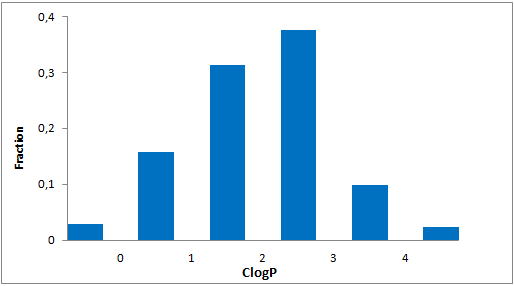
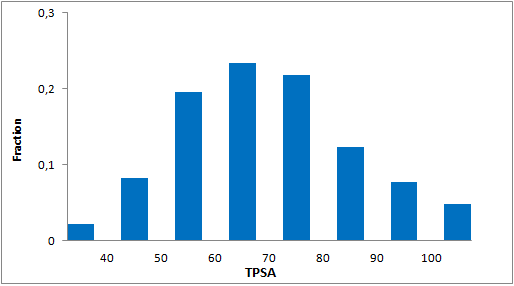
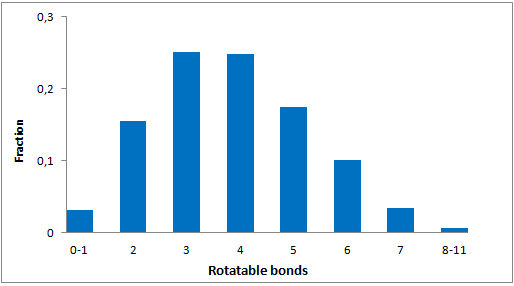
Library design
The main criterion of the selection was presence of the electrophilic groups (cyano, epoxide, carbonyl etc.), which can provide the key interaction with serine in the active site of targeted enzyme. Next we estimated the 3D-shape of the molecules and compared them by throughput docking with the volume of the active sites taken from X-ray data of several serine hydrolases. The compounds which did not fit to the active site were withdrawn.
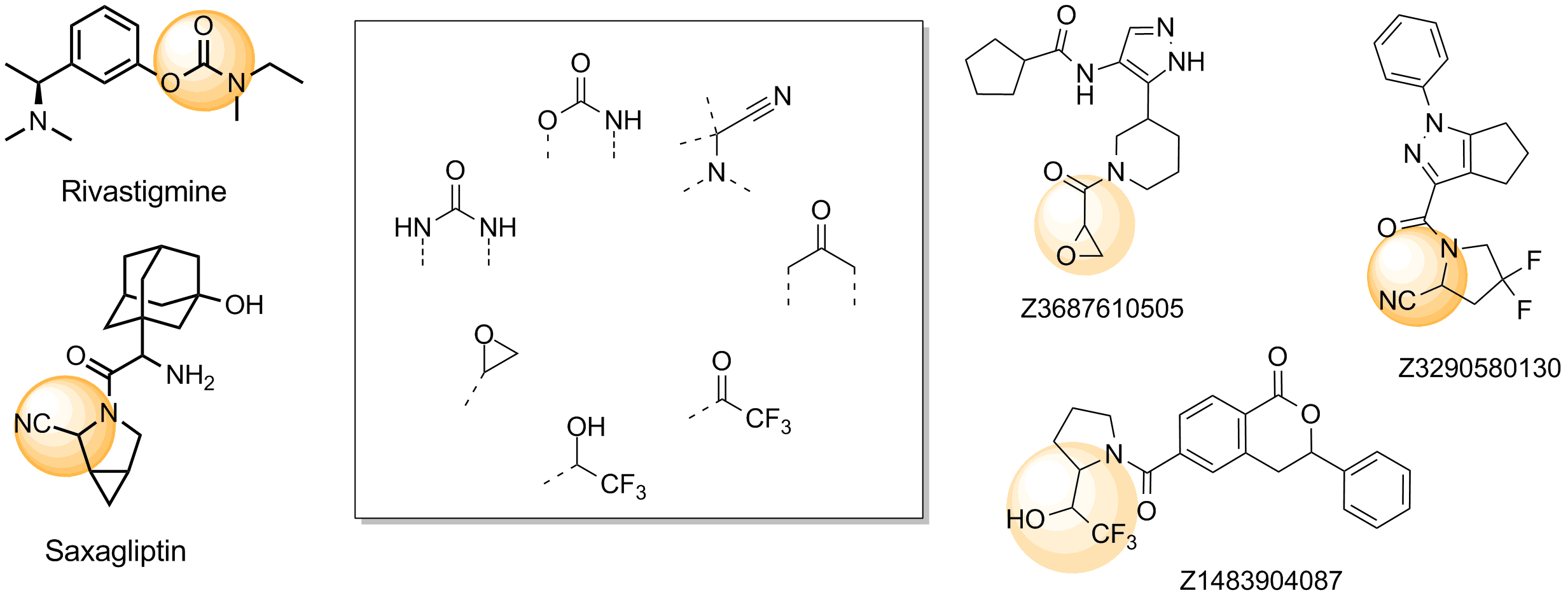
Representative examples of commercialized serine hydrolase covalent inhibitors and examples of functional groups which usually bind in the active site.
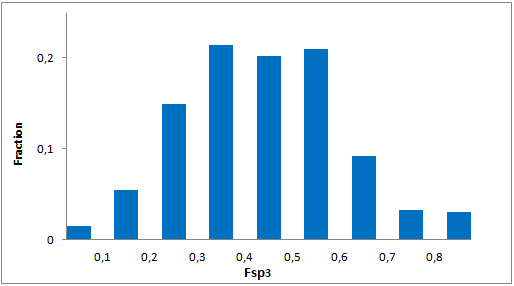
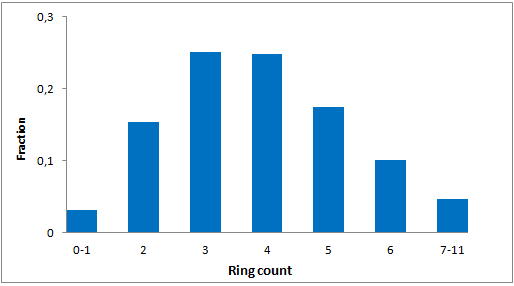
In addition, we applied several MedChem structural filters in order to remove unattractive moieties, trivial chemotypes and enhance the library with compounds with the latest scaffolds, sp3-rich frameworks and peptidomimetic structural motifs.