Designed for the discovery of new effective and safe treatment
2 500 compounds
Tuberculosis (TB) is among the most threatening diseases that can cause the next pandemic across the world. It is one of the most rampant infectious diseases owing largely to the methods of transmission and high death ratio. The absence of efficient treatment and the rapid appearance of drug resistance make TB one of the most emerging tasks to resolve in public health. Continuously mutated, Mtb remains one of the most dangerous and difficult-to-treat bacterial diseases. Lack of early diagnosis and the prevalence of resistant strains make TB extremely difficult for timely detection and treatment. Antituberculosis drug development is a high priority to prevent future pandemics. It needs further investment in scientific research and rational drug design to combat the high death rate caused by this disease.
The library is available in pre-plated formats for quick access and is supported with multiple benefits on hit follow-up studies:
- Analogs and hit samples resupply from dry stock of over 4.4 M compounds with immediate QC check.
- MedChem support in hit follow-up and optimization to lead series.
- Same-day ADME tests for design and synthesis prioritization.
Typical Formats
Catalog No.
ATB-2500-10-Y-10
Compounds
2 500
14 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well plates Greiner #781280, 320 compounds per plate, first two and last two columns empty
Price
Catalog No.
ATB-2500-50-X-10
Compounds
2 500
53 plates
Amount
50 µL of 10 mM DMSO solutions
Plates and formats
96-well plates, Greiner #650160, 80 compounds per plate, 1 & 12 columns empty
Price
Catalog No.
Library & follow-up package
Plates and formats
ATB-2500-10-Y-10 library, hit resupply from dry stock and fresh DMSO solutions plus 240+ analogs from stock and follow-up synthesis from REAL Space
Price
*We will happily provide our library in any other most convenient format for your project. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or, Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400) or send your preferred labware. Compounds pooling can be provided upon request.
Download SD file
Library Design
To design the library and select the most promising molecules, we pay attention to the complexity of Mtb cell-wall and the difficulties of membrane penetration. Despite the absence of certain ruleless and defined filters, we tried to remove all compounds that could have issues with penetration based on the reported data and structure analysis of cell active vs inactive compounds.
Having studied some special features related to the biochemical pathways and morphological properties inherent to Mtb, a group of protein targets has been selected and prioritized to perform in silico screening (vHTS) and select compounds with the alleged activity. Generally, the protein targets in this library can be divided into two subsets: Cell Wall Synthesis-associated proteins and Mtb-specific Essential protein targets distinct from those present in eukaryotic organisms.
Alanine racemase (Alr), which catalyzes the interconversion of L-alanine and D-alanine. Which in turn plays a key role in peptidoglycan cross-linking.
dTDP-4-dehydrorhamnose 3,5-epimerase (RmlC) is involved in the biosynthesis of the dTDP-L-rhamnose which is required for connection of the galactan region of arabinogalactan to peptidoglycan molecule.
Enoyl-[acyl-carrier-protein] reductase [NADH] (InhA) is involved in the biosynthesis of mycolic acids playing a key role in the fatty acid elongation process.
Decaprenylphosphoryl-beta-D-ribose oxidase (Dpre1) is a component of the complex that catalyzes the formation of decaprenyl-phospho-arabinose (DPA), a key precursor required for the synthesis of cell-wall arabinans.
N-Acetylglucosamine-1-phosphate uridyltransferase (GLMU) is an enzyme that catalyzes the final two steps in the biosynthesis of UDP-GlcNAc and is essential for the synthesis of the lipid A of lipopolysaccharide (LPS).
Shikimate kinase (AroK) is necessary for the synthesis of the common precursor of aromatic amino acids and secondary metabolites by phosphorylation of the 3-hydroxyl group of shikimic acid using ATP. The amino acids biosynthesis is also a very attractive direction for drug design, particularly as it is represented with a broad family of enzymes.
Beta-lactamase responsible for resistance to beta-lactam antibiotics.
Isocitrate lyase is in charge of the first step of the glyoxylate shunt, namely the synthesis of C4 dicarboxylic acids from C2 compounds.
ATPases (ClpC, ClpX) – chaperon proteins that induce degradation of proteins by bacterial proteasome. Play an essential role in bacterial proteostasis.
Mtb regulatory Serine Proteases (ClpP1P2) – serine proteases that form bacterial proteasomes and are responsible for protein homeostasis and degradation in bacteria.
Peptidyl tRNA hydrolase (Pth) is an essential Mtb-specific RNA hydrolase that participte in bacteria homeostasis and protein synthesis control.
The vHTS procedure assumes the application of grid-based representation of the binding site and designation of constraints based on literature data (mutation experiments) or ligand-protein interaction map with subsequent partial or full matching. Besides the rating of compounds by empirical docking score, the result conformations were also visually checked for the reliability of the binding mode. At the same time, the number of reference compounds against selected targets is relatively small and possesses a low degree of diversity inside each target-based reference set.
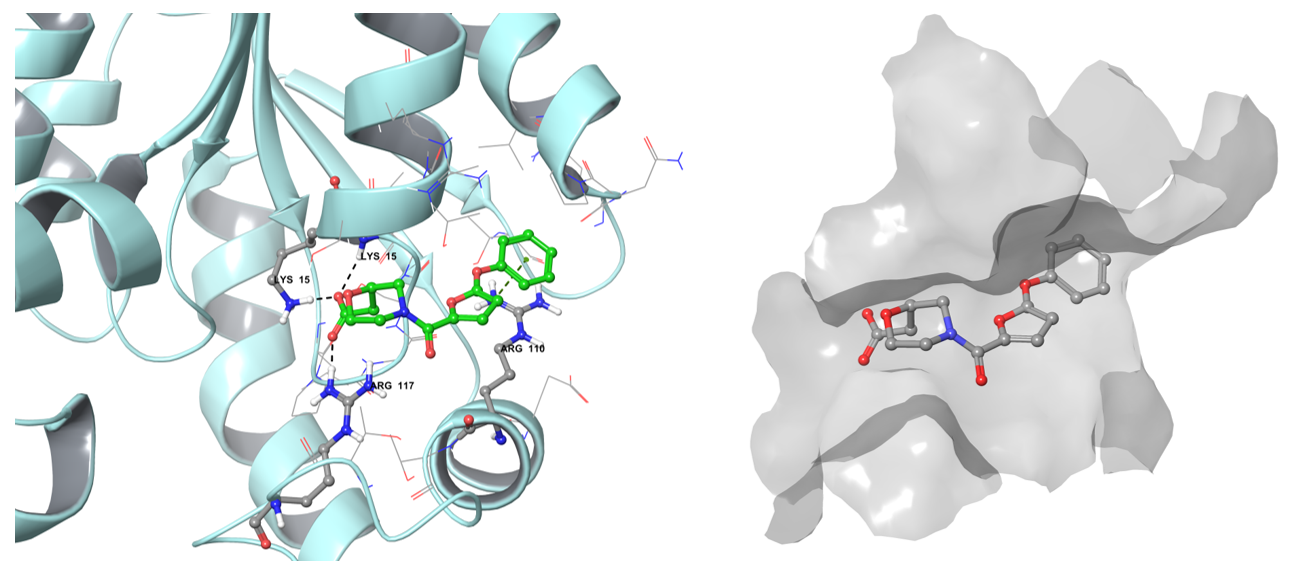
The example of the binding pose of Z1603549506 in the Shikimate kinase binding site.
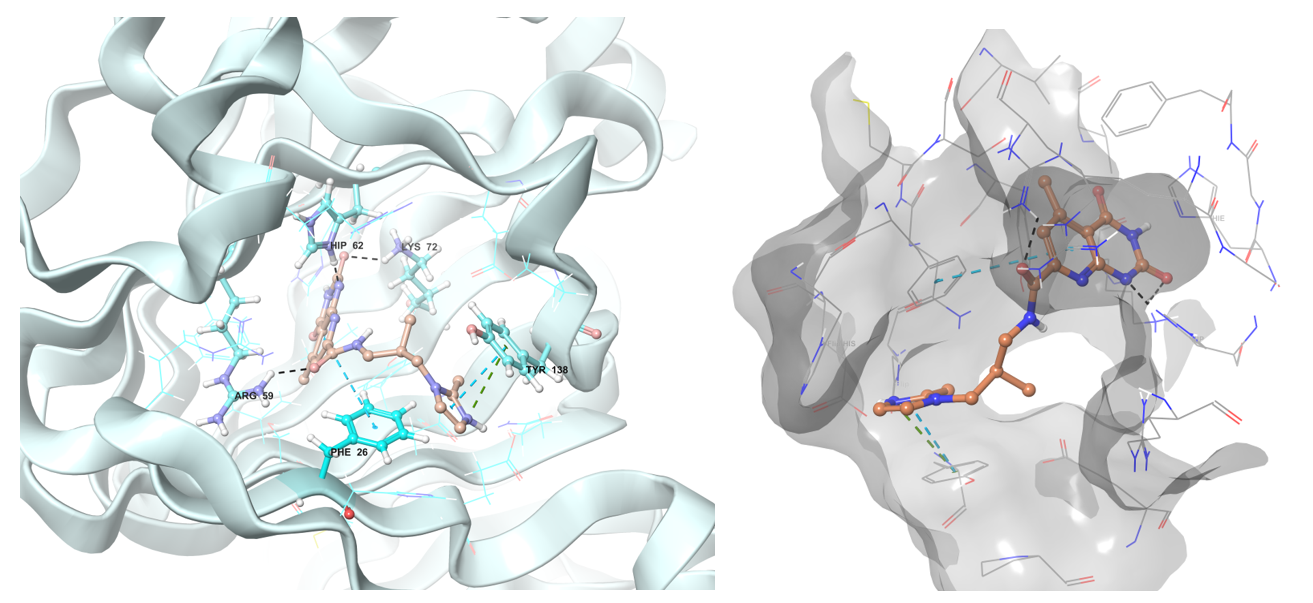
Predicted binding conformation in ribbon and shape protein representation of Z1313265676 to RmlC reductase.
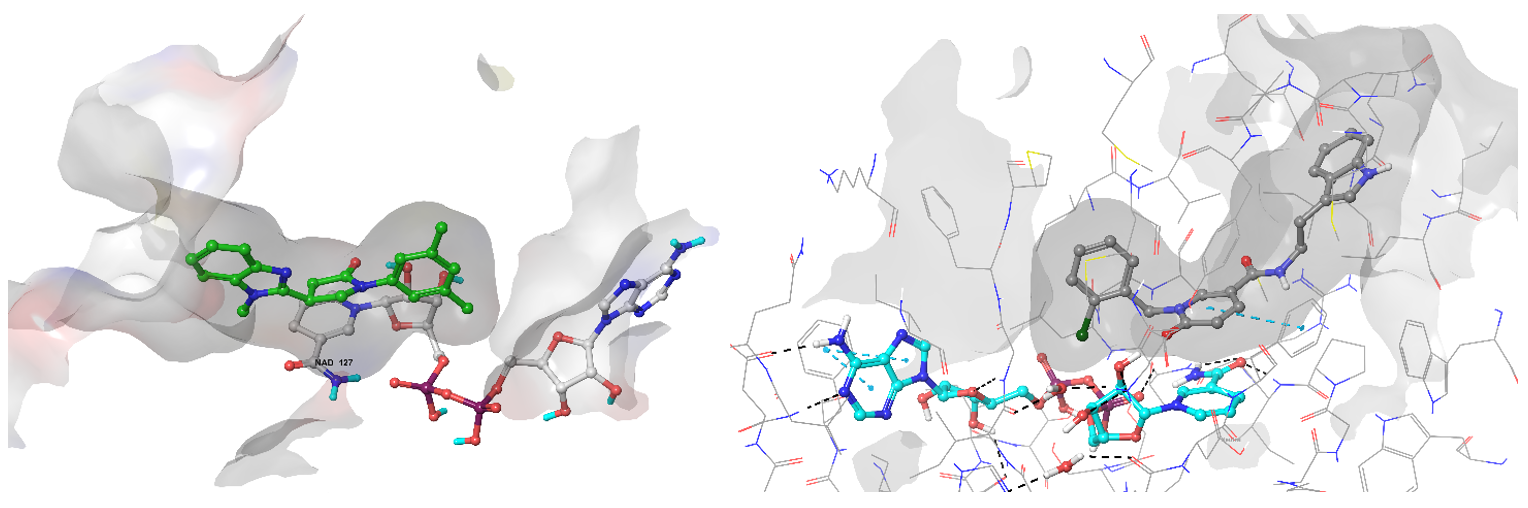
Examples of predicted hits Z111780734 (left) and Z189105794 binding conformation in the active site of InhA enzyme.
References
-
Consolidated Guidelines on Drug-resistant Tuberculosis Treatment
World Health Organization, 2019. Available online: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ -
In Silico Strategies in Tuberculosis Drug Discovery
Macalino, S. J. Y.; Billones, J. B.; Organo, V. G.; Carrillo, M. C. O. Molecules2020, 25(3), 665. doi:10.3390/molecules25030665 -
Drug Resistance Characteristics of Mycobacterium tuberculosis Isolates From Patients With Tuberculosis to 12 Antituberculous Drugs in China
Wu, X.; Yang, J.; Tan, G.; Liu, H.; Liu, Y.; Guo, Y.; Gao, R.; Wan, B.; Yu, F. Front. Cell Infect. Microbiol.2019, 9, 345. doi:10.3389/fcimb.2019.00345 -
Mycobacterium enoyl acyl carrier protein reductase (InhA): A key target for antitubercular drug discovery
Prasad, M. S.; Bhole, R. P.; Khedekar, P. B.; Chikhale, R. V. Bioorg. Chem.2021, 115, 105242. doi:10.1016/j.bioorg.2021.105242 -
Structural Basis for Inhibition of Enoyl-[Acyl Carrier Protein] Reductase (InhA) from Mycobacterium tuberculosis
de Ávila, M. B.; Bitencourt-Ferreira, G.; de Azevedo, W. F. Curr. Med. Chem.2020, 27(5), 745-759. doi:10.2174/0929867326666181203125229 -
Discovery of New and Potent InhA Inhibitors as Anti-tuberculosis Agents: Structure Based Virtual Screening Validated by Biological Assays and X-ray Crystallography
Kamsri, P.; Hanwarinroj, C.; Phusi, N.; Punkvang, A. J. Chem. Inf. Model.2019, doi:10.1021/acs.jcim.9b00918 -
New InhA Inhibitors Based on Expanded Triclosan and Di-Triclosan Analogues to Develop a New Treatment for Tuberculosis
Chetty, S.; Armstrong, T.; Sharma Kharkwal, S. Pharmaceuticals2021, 14(4), 361. doi:10.3390/ph14040361 -
Drug discovery in tuberculosis. New drug targets and antimycobacterial agents
Campaniço, A.; Moreira, R.; Lopes, F. Eur. J. Med. Chem.2018, 150, 525-545. doi:10.1016/j.ejmech.2018.03.020 -
Recent Progress in the Development of Novel Mycobacterium Cell Wall Inhibitor to Combat Drug-Resistant Tuberculosis
Belete, T. M. Microbiol. Insights2022. doi:10.1177/11786361221099878 -
Virtual screening for identifying a putative inhibitor of rmlc, a major target protein in tuberculosis disease
Sunilkumar, B.; Basheera, S. Int. J. Pharma Bio Sci.2015, 6, 616-628. -
Spinning sugars in antigen biosynthesis: characterization of the Coxiella burnetii and Streptomyces griseus TDP-sugar epimerases
Cross, A.; Roy, S. J. Biol. Chem.2022, 298(5), 101903. doi:10.1016/j.jbc.2022.101903 -
High-resolution structures of RmlC from Streptococcus suis in complex with substrate analogs locate the active site of this class of enzyme
Dong, C.; Major, L. L.; Allen, A.; Blankenfeldt, W. Structure2003, 11(6), 715-723. doi:10.1016/s0969-2126(03)00098-4 -
Synthetic molecules as DprE1 inhibitors: A patent review
Imran, M.; A S, A.; Thabet, H. K.; Abida; Bakht, M. A. Expert Opin. Ther. Pat.2021, 31(8), 759-772. doi:10.1080/13543776.2021.1902990 -
Inhibiting Mycobacterium tuberculosis within and without
Cole, S. T. Philos. Trans. R. Soc. B2016, 371, 20150506. doi:10.1098/rstb.2015.0506 -
Advances in Key Drug Target Identification and New Drug Development for Tuberculosis
Mi, J.; Gong, W.; Wu, X. Biomed. Res. Int.2022, Article ID 5099312. doi:10.1155/2022/5099312 -
Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages
Rengarajan, J.; Bloom, B. R.; Rubin, E. J. Proc. Natl. Acad. Sci. U. S. A.2005, 102(23), 8327-8332. doi:10.1073/pnas.0503272102 -
Identification of cell wall synthesis inhibitors active against Mycobacterium tuberculosis by competitive activity-based protein profiling
Li, M.; Patel, H. V.; Cognetta, A. B. 3rd; et al. Cell Chem. Biol.2022, 29(5), 883-896.e5. doi:10.1016/j.chembiol.2021.09.002 -
Characterization of M. tuberculosis SerB2, an essential HAD-family phosphatase, reveals novel properties
Yadav, G. P.; Shree, S.; Maurya, R.; et al. PLoS One2014, 9(12), e115409. doi:10.1371/journal.pone.0115409 -
High throughput screen identifies small molecule inhibitors specific for Mycobacterium tuberculosis phosphoserine phosphatase
Arora, G.; Tiwari, P.; Mandal, R. S.; et al. J. Biol. Chem.2014, 289(36), 25149-25165. doi:10.1074/jbc.M114.597682 -
Regulatory Mechanism of Mycobacterium tuberculosis Phosphoserine Phosphatase SerB2
Grant, G. A. Biochemistry2017, 56(49), 6481-6490. doi:10.1021/acs.biochem.7b01082 -
Targeting the Serine Pathway: A Promising Approach against Tuberculosis?
Haufroid, M.; Wouters, J. Pharmaceuticals2019, 12(2), 66. doi:10.3390/ph12020066 -
Characterization of M. tuberculosis SerB2, an essential HAD-family phosphatase, reveals novel properties
Yadav, G. P.; Shree, S.; Maurya, R.; et al. PLoS One2014, 9(12), e115409. doi:10.1371/journal.pone.0115409 -
Identification of cell wall synthesis inhibitors active against Mycobacterium tuberculosis by competitive activity-based protein profiling
Li, M.; Patel, H. V.; Cognetta, A. B. 3rd; et al. Cell Chem. Biol.2022, 29(5), 883-896.e5. doi:10.1016/j.chembiol.2021.09.002
Designed for the discovery of new effective modulators of Wnt/β-catenin signaling pathway
10 560 compounds
The dysregulation of Wnt/β-catenin pathway is often a key reason for cancer and other serious diseases. Wnt signaling is a highly conserved pathway that controls all steps of embryo development1-6 and tissue maintenance in mature organisms8-11. Alzheimer's, multiple sclerosis (MS), polycystic kidney disease (PKD), type 2 diabetes, arthritis, colon cancer, lung and breast cancer are some examples of Wnt pathway dysfunction. Recent evidence suggests that activation of Wnt pathway may prevent osteogenesis17,30,31 and neurodegenerative disorders.18,32
To create the ultimate Wnt/β-catenin signaling modulators library, we focused on the essential and clinically relevant protein targets. We used all available structural information and activity data for the following targets: CK1α, GSK3α/β, frizzled (Fzd) receptors and Lrp co-receptors (Lrp5/6), zinc and ring finger proteins (Znrf3, Rnf43), DKK1, Dvl-Axin, β-Catenin/TCF4, β-Catenin/Bcl9 and Bcl9/PYGO/His3 interaction inhibitions and Tankyrase to assemble the library.
The library of potential Wnt signaling modulators has been pre-plated for the most convenient and quick access. Using our Wnt library you receive multiple benefits, allowing you to save on time and costs in hit exploration and follow-up optimization:
- Hit confirmation support: Analogs search and hit samples resupply from same batch with strict QC
- Straightforward & affordable synthesis of hit follow-up libraries by parallel chemistry
- Medicinal chemistry support enhanced with on-site broad ADME/T panel
Typical Formats
Catalog No.
WPL-10-0-Z-10
Compounds
10 560
9 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
WPL-10-10-Y-10
Compounds
10 560
33 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo LDV microplates #001-12782 (LP-0200), 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
WPL-10-50-Y-10
Compounds
10 560
33 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
*We will happily provide our library in any other most convenient format for your project. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or, Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400) or send your preferred labware. Compounds pooling can be provided upon request.
Download SD file
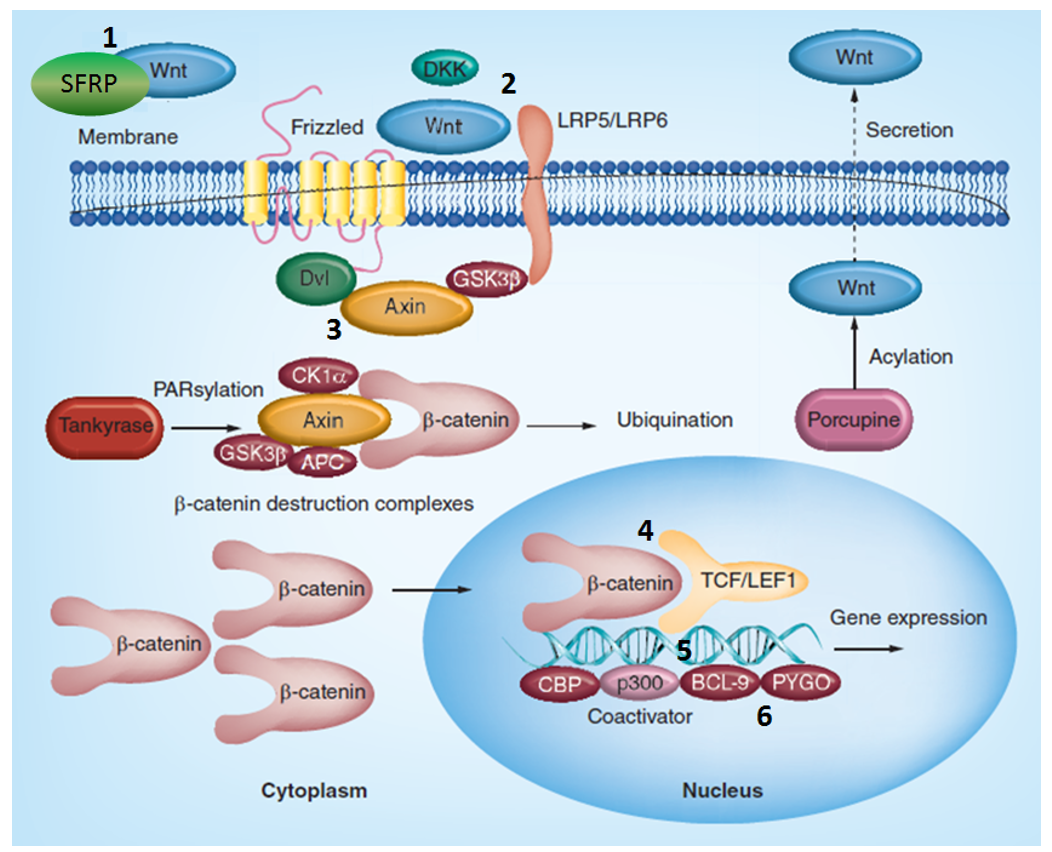
Figure 1. Canonical Wnt/Fz signaling cascade (adopted from Future Med. Chem. (2015), 7(18), 2485–2505) showing specific target-biased focused libraries available from Enamine.
Whereas numerous publications describe both identification and clinical development of Wnt inhibitors as potential anti-tumor therapies,19,20 limited number of reports deal with the therapeutic utility of Wnt activators and/or potentiators.21 These classes of small molecule modulators usually inhibit kinases implicated in stability of β-catenin, namely GSK3β and CK1. For example, CHIR-99021 (CT99021) is a GSK-3α/βinhibitor with IC50 of 10 nM/6.7 nM and > 500-fold selectivity for GSK-3 versus its closest homologs CDC2 and ERK2, as well as other protein kinases. Administration of CHIR-99021 significantly augmented hematopoietic repopulation in recipient mice transplanted with mouse or human hematopoietic stem cells (HSCs).22 This approach induces Wnt target gene expression via affecting the scaffolding proteins APC and Axin. However, GSK3 is involved in numerous signaling events in the cell and may lead to a mechanism-based toxicity. PF-670462 is a potent (IC50 = 7.7 ± 2.2 nM) and selective (>30-fold with respect to 42 additional kinases) inhibitor of CK1ε that phosphorylates Dvl protein in the Wnt signaling.23
There is the ongoing need for inhibitors, activators and potentiators (i.e. agents that mediate and/or enhance the effect of endogenous Wnt ligands) of Wnt signaling with novel mechanism of action. We combined our novel chemistries and structure-based approach to assemble a library of ca. 10,000 lead-like compounds able to tackle various aspects of Wnt/Fz signaling. Specific targets representing Wnt/Fz cascade include sFRP1, Dkk1, DyrK1A/B and interfaces of β-catenin with several transcription factors including Tcf-4 and Bcl-9. With this library we allow using either target-based, reporter-based or phenotypic assay(s) to test these compounds. Identified hits are expected to be tractable (i.e. feasible SAR and lead generation) and suitable as entry points into developing agents for the treatment of tumors, bone disease, Cardiovasular (CV) /metabolic, gastrointestinal (GI), and CNS/neurodegenerative disorders.
Specific targets and nodules of Fz/Wnt signaling cascade used for the library design:
Wnt/sFRP-1 interaction inhibitors. sFRP-1 family of proteins includes endogenous molecules that bind both Wnt ligands and Fz receptors. Inhibition of sFRP-1 may result in vicarious activation of Wnt signaling. In addition, WAY 316606 is a sFRP-1 inhibitor (Ki = 80 nM) that was described to increase total bone area in a murine calvarial organ culture assay at <1 nM concentration24. It was also reported to be an antiosteoporotic agent25. WAY-262611 is a Wnt pathway agonist that increases bone formation rate with EC50 of 0.63 uM in TCF-Luciferase assay. WAY-262611 exhibits good pharmacokinetic properties and a dose-dependent increase in the trabecular bone formation rate in ovariectomized rats following oral administration26. Specific attention needs to be paid to monitoring specific organ toxicity associated with a long-term perturbation of Wnt pathway including neoplasia(s),27,28 although recent data suggest otherwise29. This selection is represented by ca 1200 compounds.
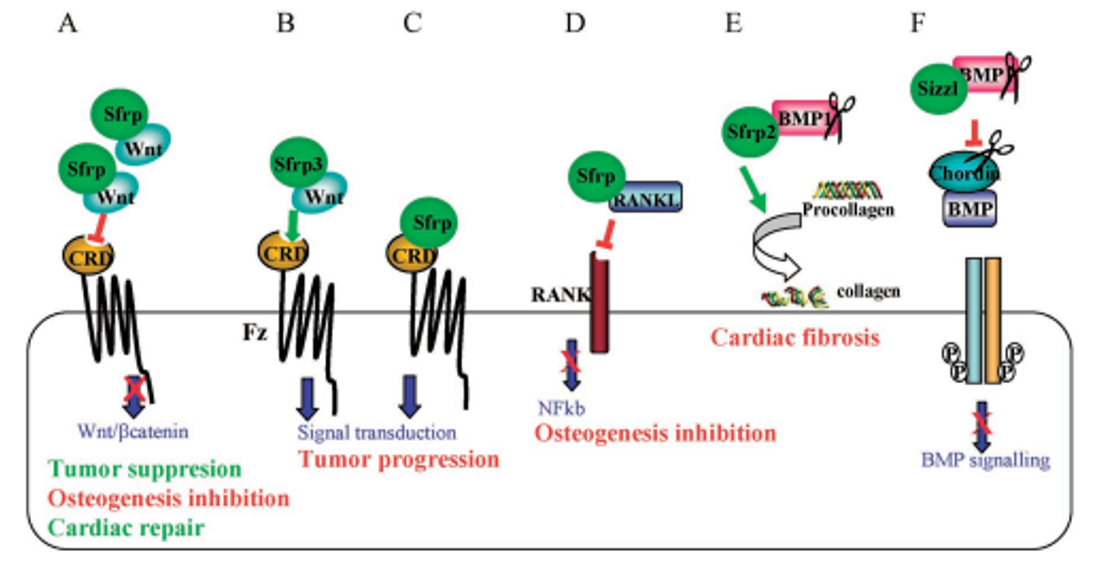
Figure 2. Multifunctional molecular interactions of sFRP1/2 and their implications in pathological events. A) sFRP1/2 sequester Wnts, thereby antagonizing Wnt/β-catenin signaling; B) Frzb (sFRP3) and Crescent favor the diffusion of Wnts, enhancing their signaling range; C) sFRP1/2 cind to Fz receptors activating intracellular signaling; D) sFRP1 binds RANKL preventing its interaction with RANK receptor; E) sFRP2 binds to and enhances the procollagen proteinase activity of BMP1/Tollpid-like metalloproteinases accelerating the processing of pro-collagen; F) Sizzled binds to and inhibits the Chordinase activity of BMP1/Tolloid-like metalloproteinase; unproteced Chordin binds and sequesters BMPs (from Tohoku J. Exp. Med. 2010, 221, 11-17).
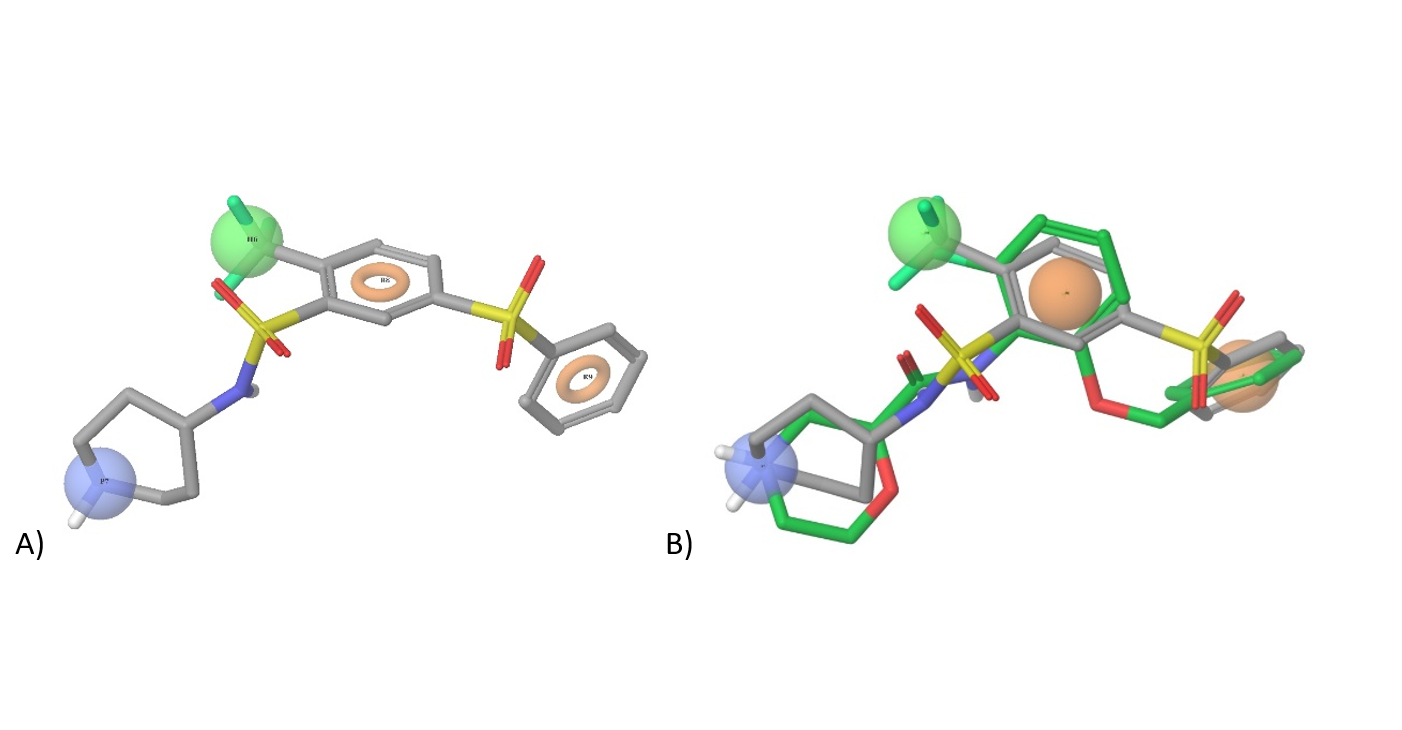
Figure 3. A) Pharmacophore model based on WAY 316606; orange – aromatic/heteroaromatic groups, green-hydrophobic pharmacophore (halogen, CF3, CHF2, Me, Et, iPr etc.), blue-positively charged moiety (+NH3Alk, +NH2Alk2, +NHAlk3 etc.); B) Superposition of WAY-316606 and our pharmacophore construct used in (sub) library design
DKK-1 Inhibitors. We used both docking and ligand-based approaches to search for potential DKK-1 binders. Specifically, the DKK1/LRP6 protein-protein interaction (PPI) interface was utilized to select matching ligands from the entire Enamine Screening Collection. The analogs of NCI8642 and other reported DKK-1/LRP6 interaction inhibitors were used for ligand-based search and pharmacophore modeling. In the docking calculation, we focused on topological and charge distribution features of the critical Dkk-1 loop that binds to LRP6. We reasoned that blocking/destabilizing this loop feature of the DKK-1/LRP6 PPI may yield modulators and/or activators of Wnt signaling while maintaining ‘normal’ Wnt-Fz-LRP6 signaling.
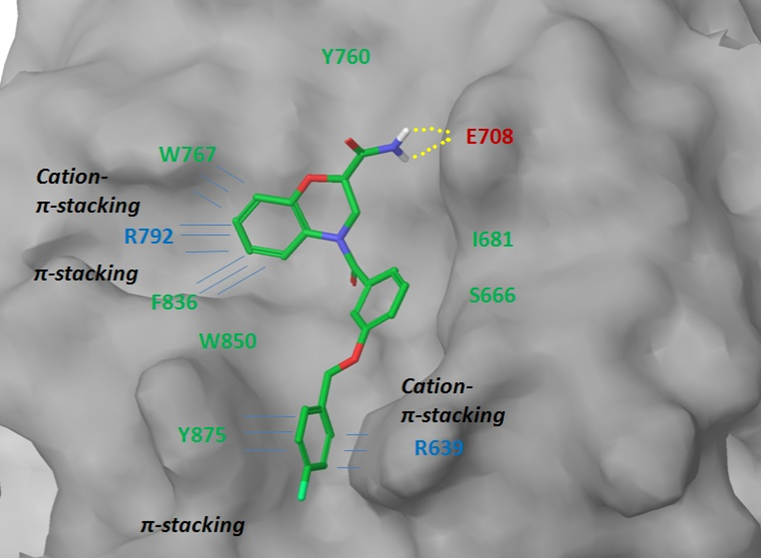
Figure 4. Binding interface between LRP6 protein and representative ligand from the focused subset.
We used known DKK-1 inhibitor WAY 262611 and its 3D pharmacophore in the ligand-based approach to identify 3k+ lead-like compounds as potential binders.
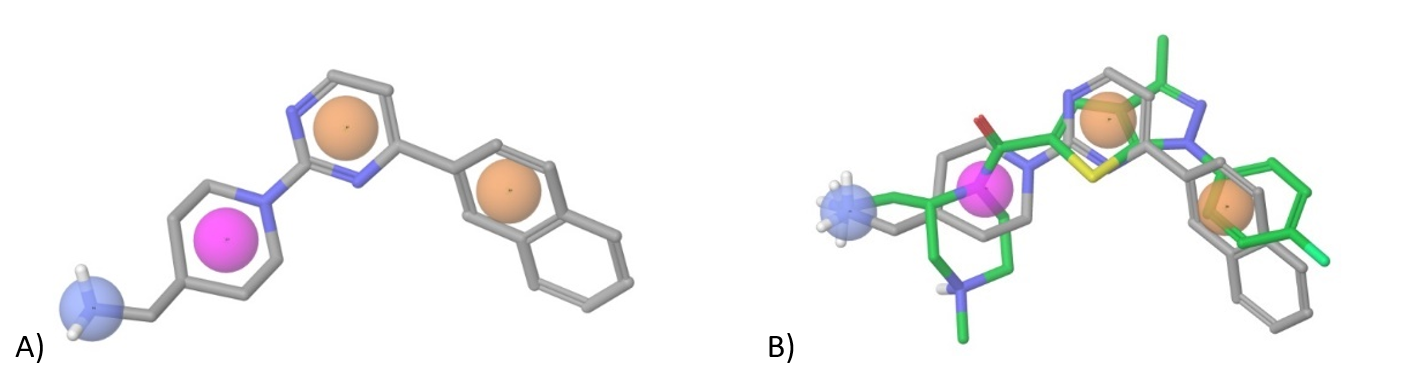
Figure 5. A) Pharmacophore model based on WAY-262611; orange – aromatic/heteroaromatic groups, blue – positively charged moiety (+NH3Alk, +NH2Alk2, +NHAlk3 etc.), magenta – aliphatic ring; B) Superposition of WAY-262611 and our pharmacophore construct used in (sub) library design.
Dvl-Axin Inhibitors. Dvl-Axin interaction is mediated by a specific PDZ-domain. Since structural information on this PPI interface is not available, we built a homology model using AlphaFold2 and numerous SBio data on PDZ domains. The resulting model was used to run docking calculation of the entire Screening Collection and identify the most promising ‘hits’. As a result, ~2500 final Dvl-biased compounds were added to the library. A representative example of putative Dvl binder is shown below.
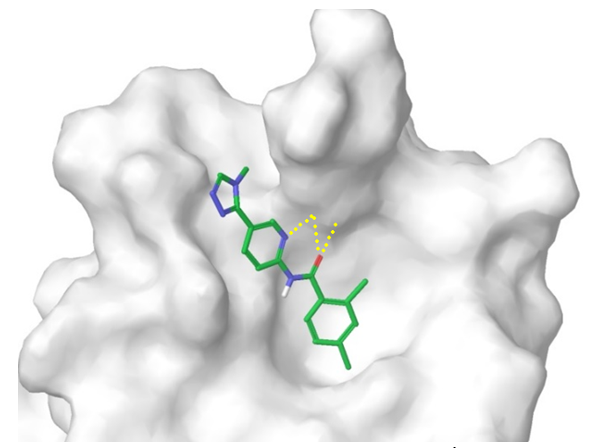
Figure 6. A representative example of Dvl/PDZ-domain binding ligand from our focused set.
β-Catenin/TCF4 Protein-Protein Interaction. To model the interaction interface between β-catenin and Tcf-4 we used reported structural data (ACS Chem. Biol. 2014, 9, 193−201) to identify critical contacts including intriguing protonated pyridine (cation) – π-interaction between docked ligand and Arg474/515 tweezers. The topology of the hydrophobic pocket and H-bond of the aromatic NH2 group with protein’s Lys508 residue allowed construction of a respective pharmacophore model (Figure 7). After docking calculation and scoring cut off, a focused selection of ca. 1800 small molecules was assembled into β-Catenin/TCF4 sublibrary.
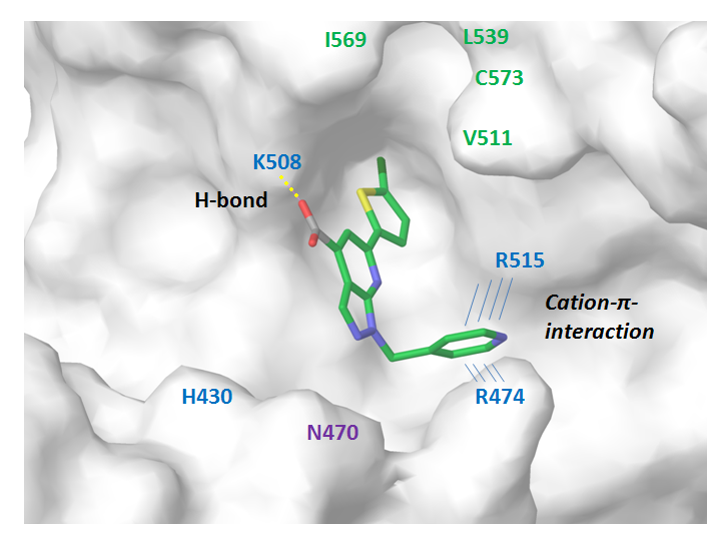
Figure 7. Binding interface between β-catenin and representative ligand from the focused subset.
β-Catenin/Bcl9 Protein-Protein Interaction Interface. We focused on the available structural data of the reported helix-helix interaction between the two biological molecules. Specifically, we investigated the key H-bonds between H358, R359 (Bcl9) and D162, D164 (β-catenin) (Figure 8). Following docking and binding poses prioritization, we picked molecules that secured hydrogen bonding with D162/D164 and exhibited a significant π-stacking/hydrophobic interaction area with β-catenin to result in the 1779-compound library.
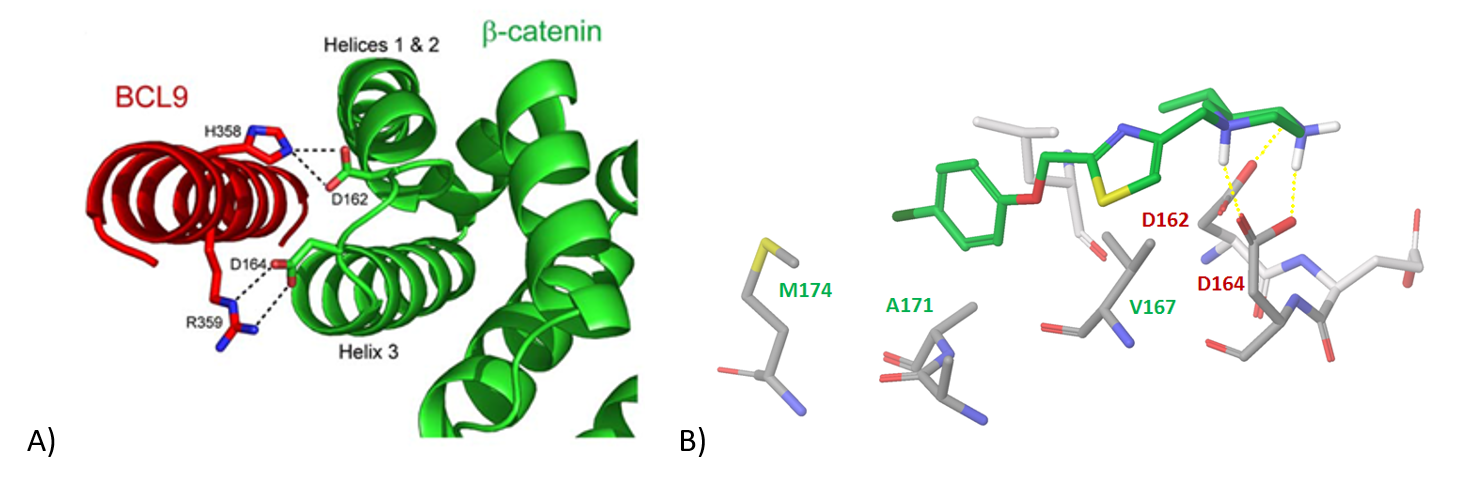
Figure 8. A) β-catenin/BCL9 helix-helix interaction interface featuring key hydrogen bonds; B) binding of β-catenin and a representative ligand from the focused subset.
Bcl9/PYGO/Histone 3 Interaction Interface. The other option to block Wnt/Fz signaling is disruption of PYGO/Histone 3 interaction (Figure 9A). Notably, it has been shown that small molecules could bind to the PHD domain of PYGO that engages the methylated Histone 3 residue, which leads to disruption of β-catenin/Bcl9 interaction. After docking calculation and scoring prioritization, 318 small molecules were selected as potential PPI inhibitors of this complex. The representative example of a ligand mediating multiple hydrophilic interactions, p-stacking with Phe366 and hydrogen bonds, is shown in Figure 9B.
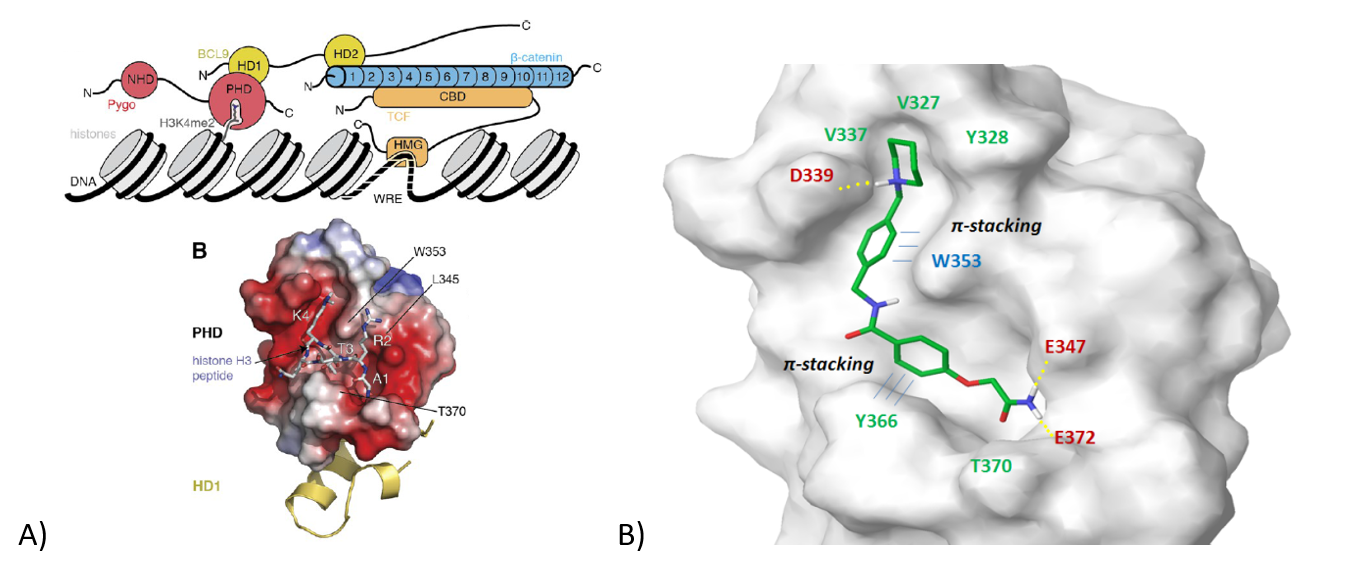
Figure 9. A) Interaction interface of BCL9/PYGO/Histone 3; B) Representative molecule bound to the key amino acids of H3K4me2.
References
-
Interaction of Wnt and Activin in Dorsal Mesoderm Induction in Xenopus
Sokol, S. Y.; Melton, D. A. Dev. Biol.1992, 154, 348-355. doi:10.1016/0012-1606(92)90054-L -
A role for maternal β-catenin in early mesoderm induction in Xenopus
Schohl, A.; Fagotto, F. EMBO J.2003, 22, 3303-3313. doi:10.1093/emboj/cdg303 -
Neural induction in Xenopus requires inhibition of Wnt/β-catenin signaling
Heeg-Truesdell, E.; LaBonne, C. Dev. Biol.2006, 298, 71-86. doi:10.1016/j.ydbio.2006.06.004 -
The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells
Hirabayashi, Y.; Itoh, Y.; Tabata, H.; Nakajima, K.; Akiyama, T.; Masuyama, N.; Gotoh, Y. Development2004, 131, 2791-2801. doi:10.1242/dev.01130 -
WNT Signaling molecules act in axis formation in the diploblastic metazoan Hydra
Hobmayer, B.; Rentzsch, F.; Kuhn, K.; Happel, C. M.; Cramer von Laue, C.; Snyder, P.; Rothbacher, U.; Holstein, T. W. Nature2000, 407, 186-189. doi:10.1038/35025063 -
Wnt/β-catenin signaling and body plan formation in mouse embryos
Marikawa, Y. Semin. Cell Dev. Biol.2006, 17, 175-184. doi:10.1016/j.semcdb.2006.02.009 -
Wnt signaling in disease and in development
Nusse, R. Cell Res.2005, 15, 28-32. doi:10.1038/sj.cr.7290260 -
A role for Wnt signaling in self-renewal of haematopoietic stem cells
Reya, T.; Duncan, A. W.; Ailles, L.; Domen, J.; Scherer, D. C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I. L. Nature2003, 423, 409-414. doi:10.1038/nature01593 -
Wnt/β-catenin Is Essential for Intestinal Homeostasis and Maintenance of Intestinal Stem Cells
Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Mol. Cell. Biol.2007, 27, 7551-7559. doi:10.1128/MCB.00104-07 -
Wnt signaling function in Alzheimer’s disease
De Ferrari, G. V.; Inestrosa, N. C. Brain Res. Rev.2000, 33, 1-12. doi:10.1016/S0165-0173(00)00018-3 -
Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration
Li, H.; Richardson, W. D. Nat. Neurosci.2009, 12, 815-817. doi:10.1038/nn.2320 -
Wnt signaling in polycystic kidney disease
Benzing, T.; Simons, M.; Walz, G. J. Am. Soc. Nephrol.2007, 18, 1389-1398. doi:10.1681/ASN.2007020158 -
Islet Specific Wnt Activation in Human Type 2 Diabetes
Lee, S. H.; Demeterco, C.; Geron, I.; Abrahamsson, A.; Levine, F.; Itkin-Ansari, P. Exp. Diabetes Res.2008, 13. doi:10.1155/2008/728763 -
Wnt signaling in rheumatoid arthritis
Sen, M. Rheumatology2005, 44, 708-713. doi:10.1093/rheumatology/keh588 -
Wnt signaling in lung cancer
Mazieres, J.; He, B.; You, L.; Xu, Z.; Jablons, D. M. Cancer Lett.2005, 222, 1-10. doi:10.1016/j.canlet.2004.09.041 -
Wnt signaling in breast cancer: have we come full circle?
Brown, A. M. C. Breast Cancer Res.2001, 3, 351-355. doi:10.1186/bcr313 -
WNT1-induced secreted protein (WISP1), a novel regulator of bone turnover and Wnt signaling
Maeda, A.; et al. J. Biol. Chem.2015, 290, 14004-14018. doi:10.1074/jbc.M115.642306 -
Deficient Wnt signaling triggers striatal synaptic degeneration and impaired motor behavior in adult mice
Galli, S.; et al. Nat. Commun.2015, 5, 4992. doi:10.1038/ncomms5992 -
Directed cardiomyogenesis of human pluripotent stem cells by modulating Wnt/β-catenin and BMP signaling with small molecules
Aguilar, J. S.; et al. Biochem. J.2015, 469, 235-241. doi:10.1042/BJ20150274 -
Identification of 2-aminopyrimidine derivatives as inhibitors of canonical Wnt signaling pathway
Del Bello, F.; et al. Bioorg. Med. Chem.2015, 23, 5725-5733. doi:10.1016/j.bmc.2015.06.052 -
Wnt/β-catenin signaling and disease
Clevers, H.; Nusse, R. Cell2012, 149, 1192-1206. doi:10.1016/j.cell.2012.05.012 -
Regulation of Wnt signaling during adipogenesis
Bennett, C. N.; et al. J. Biol. Chem.2002, 277, 30998-1004. doi:10.1074/jbc.M204527200 -
Protein kinases CK1 and CK2 as new targets for neurodegenerative diseases
Perez, D. I.; Gil, C.; Martinez, A. Med. Res. Rev.2011, 31, 924-954. doi:10.1002/med.20202 -
A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation
Bodine, P. V. N.; et al. Bone2009, 44, 1063-1068. doi:10.1016/j.bone.2009.02.011
Enamine's REAL Space represents the world’s largest, continuously expanding make-on-demand virtual library, built on insights from millions of parallel syntheses. While the vast chemical space offers incredible potential, selecting the most promising compounds for successful drug development from billions of virtual molecules can be a daunting task. That's where AI and Machine Learning come in, enabling the creation of smaller, highly focused libraries that consistently outperform traditional large HTS collections. By integrating one of Recursion's cutting-edge AI/ML tools, MatchMaker, with REAL Space, we've curated 10 powerful screening libraries from 15 000 newly synthesized compounds, specifically designed to accelerate drug discovery with precision and speed.
Recursion (NASDAQ: RXRX), is a leading clinical-stage TechBio company, leverages sophisticated machine learning to decode biology with one of the world’s largest proprietary biological and chemical datasets, enabling precision and speed in drug discovery. Recursion’s tool, MatchMaker, uses machine learning to predict small molecule compatibility with multiple protein targets, offering a scalable, less computationally intensive alternative to traditional methods. This enables faster decision-making, enhances Recursion's datasets, and pre-screens for precision modeling to accelerate drug discovery.
What Makes this Chemical Compound Library Unique and Valuable?
Most commercial compound libraries cover broad sectors of biology such as GPCRs, kinases, or antibacterials that may include hundreds to thousands of individual proteins. Here we provide targeted libraries, subsets from the Enamine REAL Space, which are designed around common features seen when grouping proteins by their binding properties. These AI-enabled libraries were built around the hundred most promising and clinically relevant drug targets grouped into 10 families.
Development Strategy
As a first step, the human proteome was encoded into binding likelihood vectors through MatchMaker, a neural network model trained on a vast collection of biochemical datasets, to predict interacting protein-ligand pairs, otherwise known as Drug-Target Interactions (DTI). The entire Enamine REAL Space library was then scanned for putative interactions within the proteome, and the resulting pairwise matrix (compound vs protein) of 2.8 quadrillion interactions was aggregated at the synthon level. The emerging space is rich in chemical and biological information. It can be navigated to identify chemo-proteomic clusters: small, focused target sets predicted to bind with structurally and chemically similar compounds.
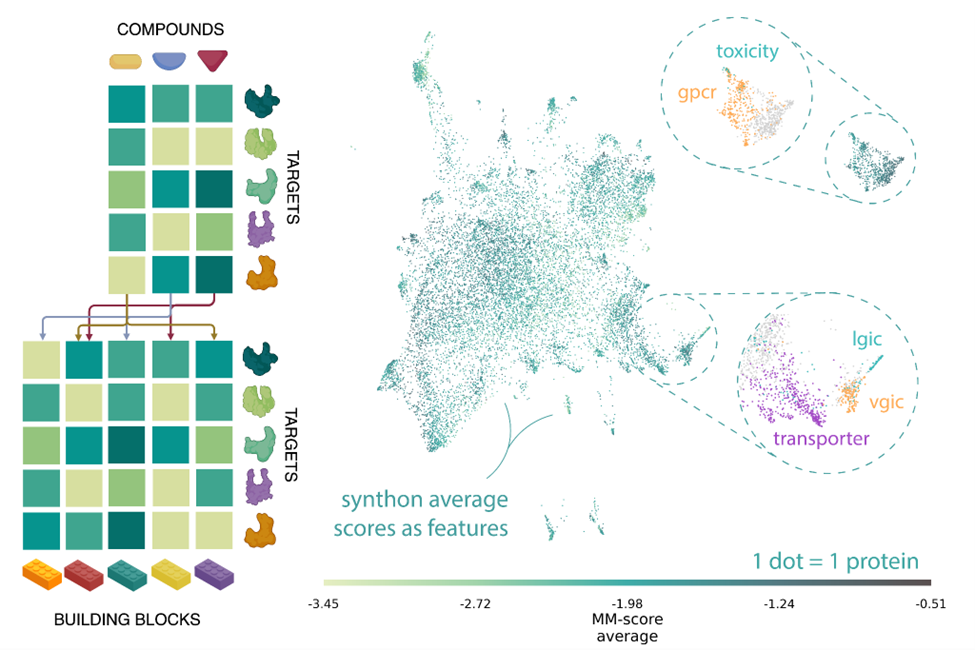
Left) MatchMaker ligand-target interaction likelihood scores were mapped onto a reduced-dimensionality space defined by synthons, theoretical building blocks of chemistry. Right) 2D projection of the human proteome as a function of their MatchMaker DTI prediction profile in synthon space. The feature space carries inherent biological and functional information as similar protein families are clustered together.
- Targeted libraries are designed starting from a list of seed targets relevant to biological pathways of interest and expanded to relevant chemo-proteomic clusters.
- Compounds were ranked against targets in each cluster according to their MatchMaker DTI prediction score.
- The final library set was selected to maximize diversity in drug-like, synthesizable chemical space, through the application of a range of classical medicinal chemistry, DMSO stability and synthesizability filters.
- 15 000 new compounds were synthesized to deliver highly focused AI-driven libraries
Libraries Catalog
Size
1 600
compounds
Description
Focused on targeting the top CRLs that have been explored as the most promising targets
Download file
Size
1 520
compounds
Description
Designed to target 3 HECT E3 Ligases for the discovery of new-generation drugs
Download file
Size
1 520
compounds
Description
Designed for discovery of RNF216, RNF19A, PRKN, RNF13 potent binders and modulators
Download file
AI-enabled USP Library
Size
1 200
compounds
Description
Designed for modulation of the largest DUBs family and delivery of a promising treatment for incurable diseases
Download file
AI-enabled GPCR Library
Size
1 760
compounds
Description
Designed to deliver new and efficient modulators of CCR5, HTR2A, HTR2B, MRGPRX1, CXCR6, CMKLR2, CCR10, GPR3, GPR4, GPR39, CCR4 receptors
Download file
HIPPO Pathway Library
Size
1 600
compounds
Description
Designed to deliver reliable hits for over 10 essential targets involved in the Hippo pathway
Download file
AI-enabled Molecular Chaperones Library
Size
1 360
compounds
Description
Designed for hit finding for Hsp90, Hsp70, Hsp60, HspD1, ClpP, Ch60 and Hsp100 protein targets
Download file
AI-enabled Allosteric Ion Channel Library
Size
2 080
compounds
Description
A new approach for modulating the most investigated and effective drug targets with good clinical records
Download file
AI-enabled PARP Library
Size
1 440
compounds
Description
Designed to target the most investigated and validated for drug discovery PARPs
Download file
AI-enabled TF Library
Size
1 520
compounds
Description
The hottest protein targets for discovering new treatments for the most challenging and not yet treated diseases
Download file
Support
We offer comprehensive support in developing your hit compounds. Naturally such programs are realised most efficiently when biological actives originate from our screening collection. However, even if the hit compounds are from the collections of other vendors lead identification and optimization projects can proceed most productively in our hands. Sometimes for this we only need to synthesize first examples of the given chemical series and validate synthesis route.
Designed to effectively target the receptor and block estrogen release
8 960 compounds
Breast cancer remains one of the major health problems among the women’s population. This disease frightens millions of women around the world. The tremendous efforts of researchers and drug developers in this field have made significant progress in treatment and early diagnostics. Breast cancer is often fueled by hormones and most commonly uses estrogen to grow. Treatment with anti-estrogen therapy can effectively block the growth of the cancer cells. Thus, Estrogen Receptor (ER) remains the most important target for the development of new effective drugs against breast cancer.
We combined different approaches in the design of ER focused library to bring the most promising and convenient for follow-up starting points in your screening campaign. The library has been carefully reviewed in the context of MedChem tractability and intrinsic high diversity. Additionally, compounds were reviewed for sufficient solubility and stability in DMSO solution to ensure the high quality of the library.
Typical Formats
Estrogen Receptor Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
ERL-8960-0-Z-10
Compounds
8 960
7 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
ERL-8960-10-Y-10
Compounds
8 960
28 plates
Amount
≤ 10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
ERL-8960-50-Y-10
Compounds
8 960
28 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
ERL-8960-10-Y-10 screening library 8 960 cmpds, hit resupply, analogs from 4.4M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD files
Library design
The database of activities related to SERMs (Selective Estrogen Receptor Modulators) was collected from the public domain (ChEMBL and Reaxys) and analyzed by the activity range. In total, about 15k activities were found, analyzed, and then separated by chemotypes. The most interesting from medchem prospective scaffolds were used for substructure searches, while the most active molecules were assembled into a validation set for in silico models – 3D pharmacophore and docking.
In addition, all available RE structures in PDB were combined and analyzed. Over 200 co-crystallized ligands with ER-alpha were extracted and superimposed to identify the most important interaction points and common structural motifs.
Superposition of all ER-alpha co-crystalized ligands, www.rcsb.org:
- All ligands share a similar binding mode which aligns with Estradiol binding.
- Two hydroxy groups from different/opposite parts of the binding pocket.
- One hydroxy group (H-bond donor) is well conserved among all extracted ligands.
- The conserved H-bond interaction with the OH-group is essential for activity when another can be replaced with different bioisosteres and H-bond donors.

About 15 distinct pharmacophores were identified with the four most common interaction maps selected for the in silico models:
- Estrogen receptor alpha 7 clusters based on PDB ID: 1sj0, 2iog, 2qxm, 3os8, 5aau, 5dwi, 5krj.
- Estrogen receptor beta 5 clusters based on PDB ID: 1nde, 1qkm, 2fsz, 2giu2yly.
Three 3D-pharmacophore models with exclusion volume were built, validated, and then used for the search of potential new ER ligands. As a result, over 10k compounds were identified as potential hits for ER-alpha, and 7k+ potentially active compounds were identified for ER-beta. The final library of potential estrogen receptor modulators passed through a manual review of predicted binding poses and was filtered using extended MedChem filters.
Examples of the pharmacophore models with reference molecules and predicted hits




Hydrophobic features are marked as orange spheres; Hydroxy group function is highlighted with purple.
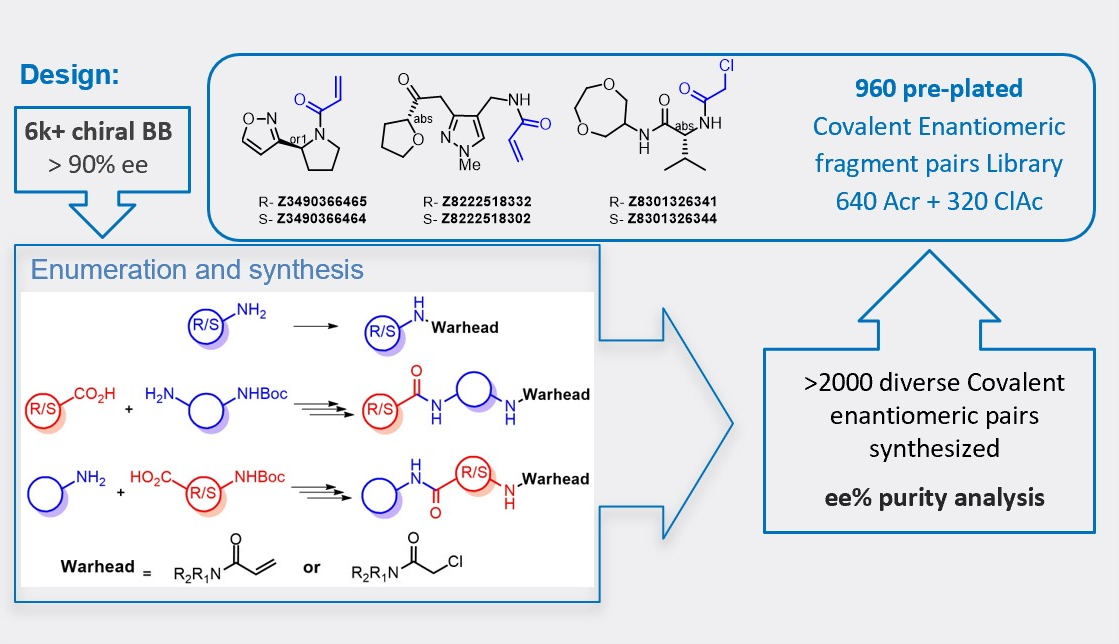
Enantiomeric pairs of covalent electrophilic fragments
960 compounds
Covalent chemical probes have become a valuable tool in drug discovery within the last few years. New technologies and development of fast mass spectrometric screening and imaging open a new horizon for proteome-wide screening and proteomics discoveries. The impressive number of successful applications brought aspiration to the discovery and synthesis of new covalent probes. Previously developed by Cravatt research group mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs makes this field especially attractive for further investigations.
To support further research of the stereoselective interaction of proteins with chiral covalent small-molecules we designed and specially synthesized two sets of fragments with the most robust covalent warheads – acrylamides and chloroacetamides.
Typical Formats
Catalog No.
CEP-960-10-Y-100
Compounds
9603 plates
Amount
10 µL of 100 mM DMSO solutions
Plates and formats
384-well echo qualified LDV microplates, 1,2 and 23, 24 columns empty, 320 compounds per plate
Price
Catalog No.
CEP-960-25-Y-20
Compounds
9603 plates
Amount
25 µL of 20 mM DMSO solutions
Plates and formats
384-well microplates, Greiner bio-one, 1, 2 and 23, 24 columns empty, 320 compounds per plate
Price
Catalog No.
CEP-960-50-X-20
Compounds
96012 plates
Amount
50 µL of 20 mM DMSO solutions
Plates and formats
96-well plates, 2D-barcoded microtubes with first and last columns empty, 80 compounds per plate
Price
Catalog No.
CEP-960
Compounds
960
Amount
Custom
Plates and formats
Any custom format
Price
Download SD file
Library code: CEP-960
Version: 3 March 2024
960 compounds
at 100 mM in DMSO
640 compounds
at 100 mM in DMSO
320 compounds
at 100 mM in DMSO
More than 6000 chiral building blocks with enantiomeric excess (ee) purity of 90%+ were analyzed and triaged for further synthesis of covalent binder pairs. The resulting set of over 2000 of the most diverse novel covalent modifiers has been synthesized. The enantiomeric purity of the corresponding enantiomeric pairs was analyzed by chiral chromatography. Compounds that passed rigorous QC and selection based on the diversity and Ro3 criteria were assembled into the Covalent Enantiomeric Pairs Library.
Key features
- Stereoselective interaction of target with enantiomeric covalent binder provides evidence of ligand-protein interaction
- Information about “correct” stereochemistry of hit on early stage
- Most common and well-validated warheads
Enatiomeric Pairs Fragment Library is plated at 100 mM concentration and is available for fast supply in different formats. The Library consists of two sublibraries, which can be acquired separately.