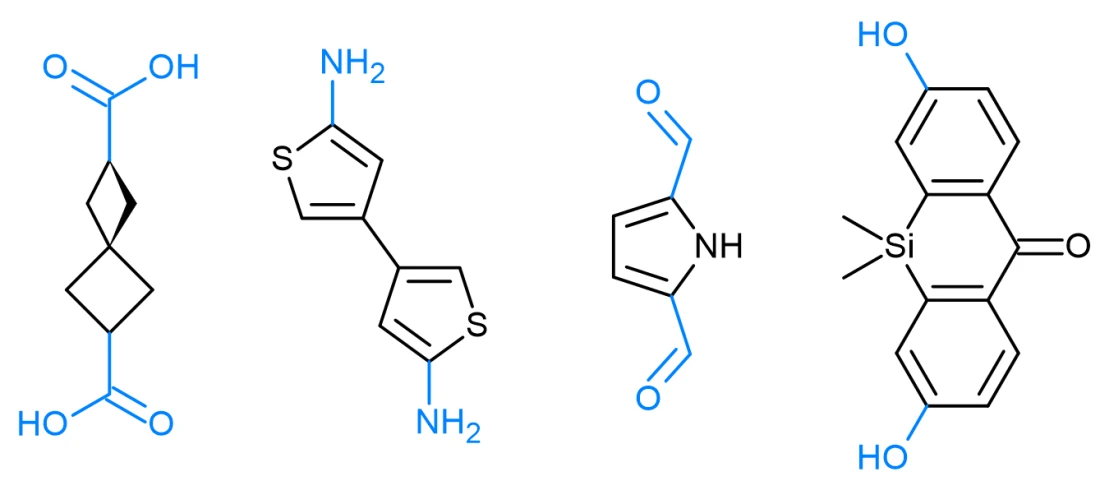
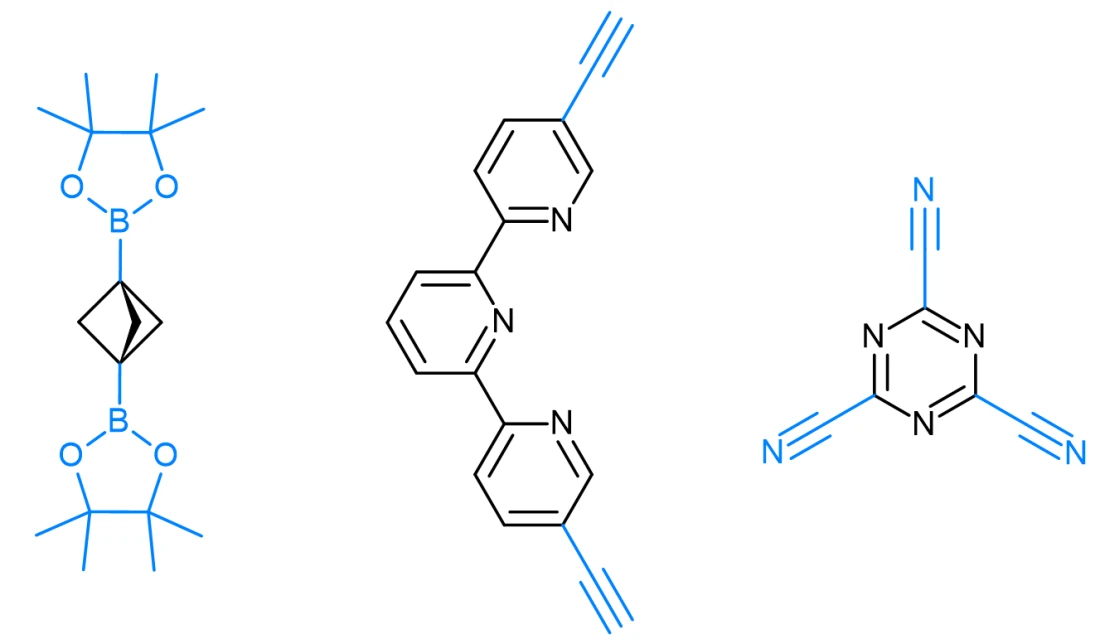
Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) are highly porous, crystalline materials designed for precise molecular interactions in areas such as gas storage, molecular separation, catalysis, and drug delivery. Their modular architectures enable fine control over pore size, surface area, and chemical environment, making them indispensable tools in contemporary materials science. The transformative impact of this class of materials was recognized with the 2025 Nobel Prize in Chemistry, awarded to Susumu Kitagawa, Richard Robson, and Omar Yaghi for their pioneering development of MOFs and their contribution to sustainable technologies.
The design and selection of linkers are central to the development of both functional MOFs and COFs, as they largely determine the resulting framework’s topology, porosity, and reactivity. Linkers can vary in several key parameters:
- Donor groups
- Connectivity (di-, tri-, tetra-, or multitopic)
- Size and linearity
- Symmetry
- Functionalization with additional organic substituents
We provide an extensive range of linkers for MOF and COF synthesis, readily available from stock.
- For MOFs: N-heterocyclic linkers, aldehyde linkers, amine linkers, carboxylic acids, hydroxy linkers, and monodentate nitrogen ligands.
- For COFs: Alkynyl linkers, amine linkers, boronic and borate linkers, nitrile linkers, and nitro linkers.
In addition to our comprehensive catalog, we also provide custom synthesis, ensuring solutions precisely aligned with your research objectives.
Examples of Enamine linkers for MOFs and COFs:
C-H Activation Catalysts
C-H activation catalysts are innovate organometallic complexes and organic compounds that enable the direct functionalization of carbon-hydrogen bonds, transforming traditionally inert C-H bonds into reactive sites. Their remarkable ability to modify organic molecules with high precision has significant applications in advanced organic chemistry.
Download SD files
Chiral Catalysts
Chiral catalysts are specialized molecules or complexes that work as enantioselective catalysts and facilitate asymmetric chemical transformations preferring one of the stereoisomers. These catalysts, including metal-containing complexes with chiral ligands and organic molecules with defined stereochemistry, revolutionized the story of asymmetric synthesis. They provide highly efficient routes to optically pure compounds, which are essential for pharmaceutical development and the production of precise chemicals.
Download SD files
Cross-Coupling Catalysts
Cross-coupling catalysts, including Buchwald catalysts and precatalysts, are essential organometallic compounds in organic synthesis, enabling the formation of complex molecular structures with high precision through carbon-carbon, carbon-oxygen, and carbon-nitrogen bond formation and others. These catalysts have significantly advanced synthetic chemistry in pharmaceuticals, materials science, and advanced chemical manufacturing.
Buchwald Precatalysts
Buchwald precatalysts are advanced organometallic complexes, primarily based on palladium, designed by Prof. S. Buchwald to facilitate efficient cross-coupling reactions in organic synthesis. These precatalysts are distinguished by their ability to generate active catalytic species in situ, offering enhanced stability, storage, and handling compared to traditional catalysts.
Download SD files
Other Cross-Coupling Catalysts
Cross-coupling catalysts, including complexes based on nickel (alternative palladium) or other metals, demonstrate varied reactivity, selectivity, and efficiency. They offer diverse capabilities in organic synthesis, enabling the formation of unique C-C, C-N, C-O, and C-S bonds under a wide range of reaction conditions.
Download SD files
Hydrogenation Catalysts
Hydrogenation catalysts are modified transition metals or their compounds, such as ruthenium, palladium or nickel metalocomplexes, that facilitate the addition of hydrogen to unsaturated organic compounds, enabling key transformations in chemical synthesis.
Download SD files
Inorganic Catalysts
Inorganic catalysts are powerful materials composed of non-carbon elements that dramatically accelerate chemical reactions while remaining unchanged. These catalysts play a critical role in optimizing industrial processes—from petrochemicals to environmental applications—by enhancing reaction rates and cutting energy costs. With unmatched stability and efficiency, inorganic catalysts are key to driving innovation and sustainability in industries worldwide.
Download SD files
Low Energy Photoredox Catalysts
Low-energy photoredox catalysts include iridium- and osmium-based complexes that exhibit enhanced photosensitization in the longer-wavelength region compared to conventional photocatalysts. Iridium catalysts are highly effective in sp²-sp³ coupling, decarboxylative arylation and other photoredox transformations, offering versatile reactivity under mild conditions. Osmium catalysts, on the other hand, serve as powerful sensitizing agents in biochemical and medical research, enabling precise photoactivation in biological systems. Additionally, this category features two biotin-conjugated molecules designed for use alongside osmium catalysts in targeted studies, further expanding their applications in bioanalytical research.
Download SD files
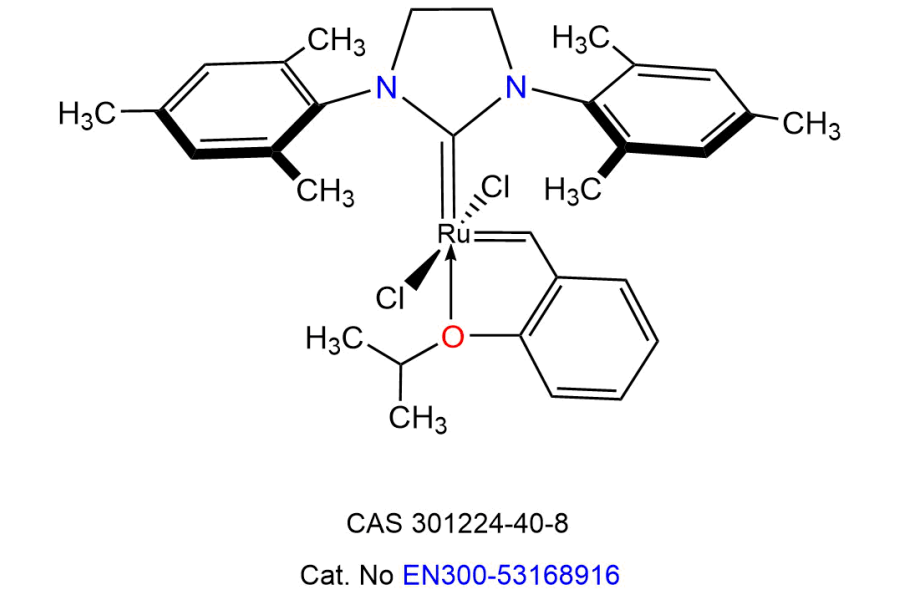
Metathesis Catalysts
Metathesis catalysts, particularly ruthenium-based complexes such as Grubbs and Hoveyda-Grubbs catalysts, represent a groundbreaking advancement in organic synthesis by enabling the redistribution of carbon-carbon double bonds. These exceptional catalysts function via a unique mechanism involving metal-carbene intermediates, facilitating a wide range of transformations, including ring-closing metathesis (RCM), cross-metathesis (CM), and ring-opening metathesis polymerization (ROMP).
Download SD files
N-Heterocyclic Carbene (NHC) Complexes
N-Heterocyclic Carbene (NHC) complexes are advanced chemical compounds featuring a central carbene unit, which is bonded to a heterocyclic ring structure. Known for their exceptional stability and reactivity,facilitating a wide range of catalytic reactions. These complexes are widely utilized in organic synthesis, pharmaceuticals, and materials science, enabling more efficient reactions with minimal side products and improved selectivity.
Download SD files
Organocatalysts
Organocatalysts are small organic molecules that effectively catalyze chemical reactions without requiring metal centers, offering unique advantages in asymmetric synthesis and green chemistry. These catalysts, including various organonitrogen, organophosphorus, and other compounds, enable stereoselective transformations with high efficiency and minimal environmental impact.
Download SD files
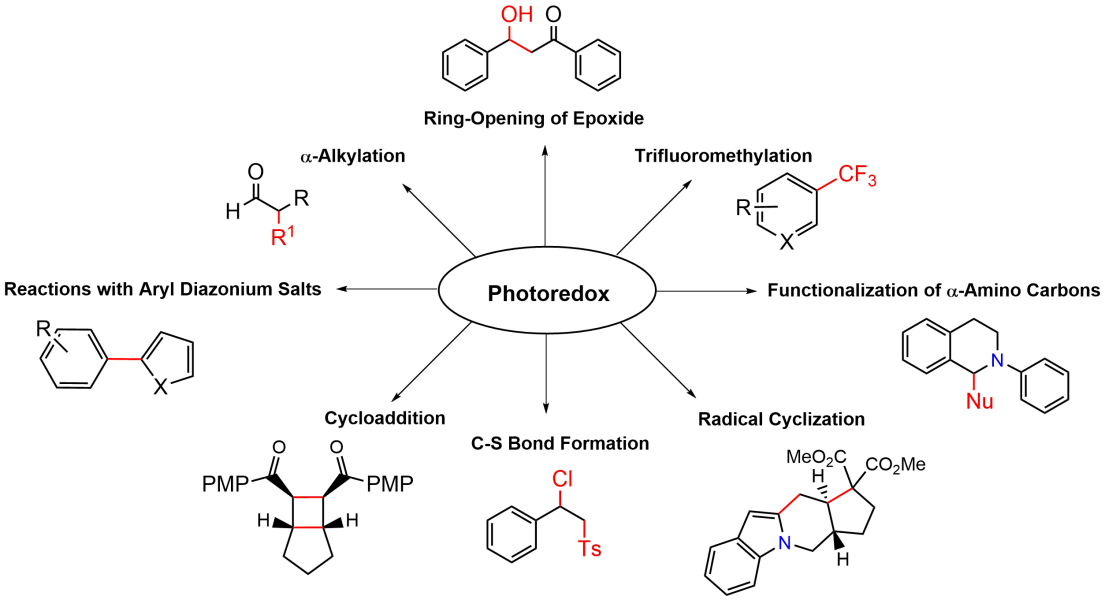
Photoredox Catalysts
Photocatalysts are specialized compounds that harness light energy to facilitate chemical transformations. These catalysts include a wide range of organic molecules and metal complexes, such as those containing ruthenium(II) and iridium(III). They enable unique reaction pathways through single-electron transfer (SET) processes and the generation of reactive intermediates under mild conditions, making them particularly valuable in environmentally friendly chemistry.
Download SD files
Transition Metal Catalysts
Transition metal catalysts, including complexes of copper, iron, cobalt, and other metals, are essential for a wide range of chemical transformations in both industrial and laboratory settings. They are especially important in hydroformylation reactions, oxidation processes, and polymerization, with broad applications in pharmaceutical synthesis, materials science, and large-scale chemical production.
Buchwald Ligands
Buchwald ligands are sterically hindered and electron-rich phosphine compounds belonging to the class of dialkylbiarylphosphines. They are highly effective in various palladium-catalyzed cross-coupling reactions and enable exceptional catalytic activity and stability under mild reaction conditions, particularly in challenging C-N, C-O, and other bond formations. The systematic modification of their biaryl backbone and phosphine substituents allows fine-tuning of their electronic and steric properties, making them valuable tools for process and method development in academic and industrial settings.
Download SD files
Cross-Coupling Ligands
Specialized organic molecules that facilitate metal-catalyzed coupling reactions. By binding to metal centers, these ligands enhance catalyst stability and regulate reaction selectivity. Common examples include phosphines and N-heterocyclic carbenes, which are indispensable in Suzuki, Buchwald-Hartwig, and other cross-coupling reactions widely employed in pharmaceutical development and materials synthesis
Download SD files
Ligands for Asymmetric Catalysis
Ligands for asymmetric catalysis include both chiral and non-chiral molecules that create specific three-dimensional environments around metal centers, facilitating the synthesis of enantiomerically pure compounds. These ligands are essential in asymmetric catalysis as they influence the spatial arrangement of reactants, guiding the course of enantioselective reactions and promoting the preferential formation of the desired stereoisomers.
Download SD files
Ligands for C-H Activation
Molecules that facilitate the selective functionalization of traditionally unreactive carbon-hydrogen bonds, by stabilizing metal catalysts and directing their activity toward specific C-H bonds. These ligands enable precise control over pathways of the C-H bond functionalization. Their distinctive electronic and steric properties render them indispensable tools in contemporary organic synthesis.
Download SD files
Ligands for Ullmann Сross-Сoupling
Ligands for Ullmann cross-coupling are organic molecules that facilitate C(sp²)-N and C(sp²)-O bond formation under copper catalysis at ambient temperature. These ligands offer a cost-effective alternative to conventional phosphine ligands and palladium catalysts commonly used in Buchwald-Hartwig cross-coupling reactions. By stabilizing copper intermediates and enhancing catalytic efficiency, they enable milder reaction conditions while maintaining high yields and selectivity. Their applications extend across various industries, including pharmaceuticals, agrochemicals, and advanced materials, where selective heteroatom coupling plays a critical role in molecular design.
Download SD files
N-Heterocyclic Carbene (NHC) Ligands
Cyclic compounds characterized by a divalent carbon atom situated between two nitrogen atoms, enabling the formation of exceptionally strong bonds with metal centers. These molecules exhibit superior thermal stability and distinctive electronic properties compared to phosphine ligands, making them indispensable in cross-coupling reactions, olefin metathesis, and a wide range of transformations in organic synthesis. Their modular design allows for precise tuning of steric and electronic properties through structural modifications, further enhancing their versatility.
Download SD files
Other Phosphine Ligands
Phosphine ligands are organophosphorus compounds that play an important role in transition metal catalysis by modulating the electronic and steric properties of metal centers. These versatile ligands form stable metal-phosphorus bonds via their lone pair of electrons, enabling precise control over catalyst reactivity and selectivity. Their tunable nature, achieved through variations in substituents on the phosphorus atom, makes them indispensable tools in modern organic synthesis and catalytic processes.
Download SD files
Photoredox Ligands
Light-sensitive molecules that facilitate electron transfer in catalytic processes. They absorb visible light to form excited states, enabling single-electron transfer (SET) pathways. This allows for mild and selective organic transformations. Photoredox chemistry becomes increasingly relevant, offering environmentally friendly and mild reaction conditions while enabling complex transformations, such as C–C bond formation, oxidative couplings, and radical-mediated processes, that are challenging to achieve using conventional methods.
Download SD files
Ligands for Other Types of Catalysis
Ligands for various types of catalysis, designed to support broad applications in research and industry. They enable enhanced reactivity and selectivity in processes ranging from organic synthesis to materials development. A versatile choice for advancing catalytic performance across diverse chemical transformations.
Download SD files
Speed, Quality, and Novelty
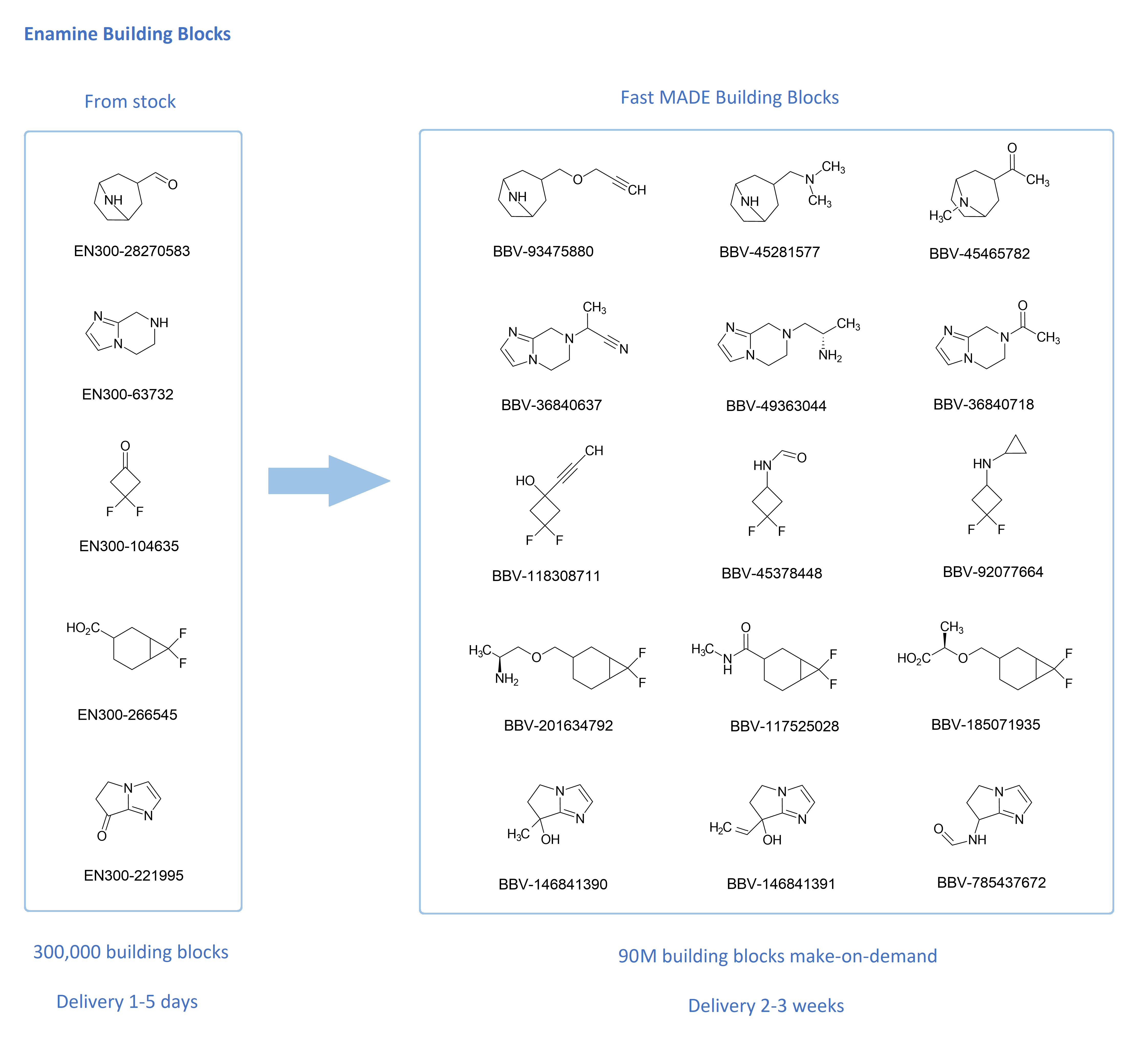
Advancements of new technologies and promising discoveries is progressing faster and more intensively with each passing day. Therefore, Enamine, a global leader in the chemical building block market, has developed a special product - the Fast MADE Building Blocks. This is a special set of Enamine MADE (MAke-on-DEmand) building blocks (BBs) that will be synthesized and delivered to our customers in ultra-short terms.
Such compounds are synthesized within 7‑10 days, through 1-3 commonly utilized procedures (Amidation, heterocyclization, arylation, Grignard reaction, reductive amidation, etc.) with a very high success rate (more than 80%). They are readily available through proven synthetic protocols using our in stock building blocks. It takes just 2‑3 weeks from the moment of the order to deliver to our customers.
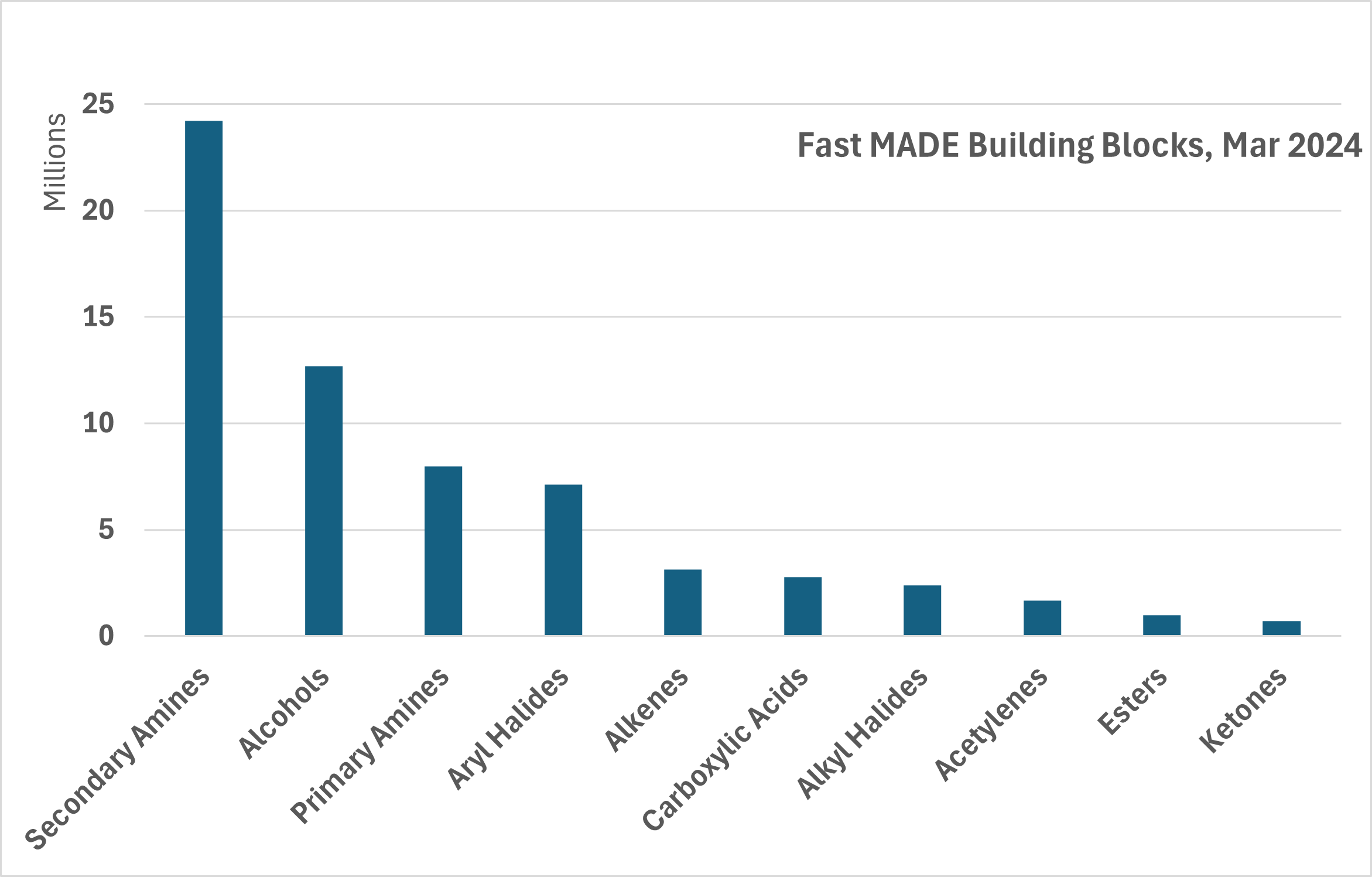
Well-validated synthesis protocols and the availability of a large amount of various starting raw materials in stock (above 300,000 BBs in gram quantities and 10,000 BBs in quantities > 1 kg), allow us quickly and easily synthesize more than 90M Fast MADE Building Blocks.
Our highly qualified and experienced specialists are constantly working to ensure that the set of the Fast MADE Building Blocks continues to grow, and synthesis protocols are being improved.
The jointly acquired broad experience now allows us to offer our customers a large number of diverse compounds. Almost all the compounds are novel, and not available through other suppliers. The Fast MADE includes diverse classes of chemical compounds, such as alcohols, primary and secondary amines, amides, sulfonyl chlorides, halides, carboxylic acids, etc.
Key Facts:
- Your ordered compounds and compound sets will be synthesized and delivered to you within a period of up to 2‑3 weeks;
- In-stock availability of raw materials directly at Enamine enabling immediate start of the synthesis;
- Well-developed, standardized, and improved synthesis protocols allow us to obtain targeted Building Blocks with very high success rates (more than 80%) in the shortest possible time;
- All of our chemical building blocks undergo extensive quality control by NMR and LC-MS, and purity is guaranteed to be over 95%.
You can conveniently make an online substructure or similarity search for novel, previously unseen compounds at our partner website: https://chem-space.com/search, the only online resource that has the comprehensive and most up-to-date collection of Fast MADE Building Blocks.
Molecular Properties
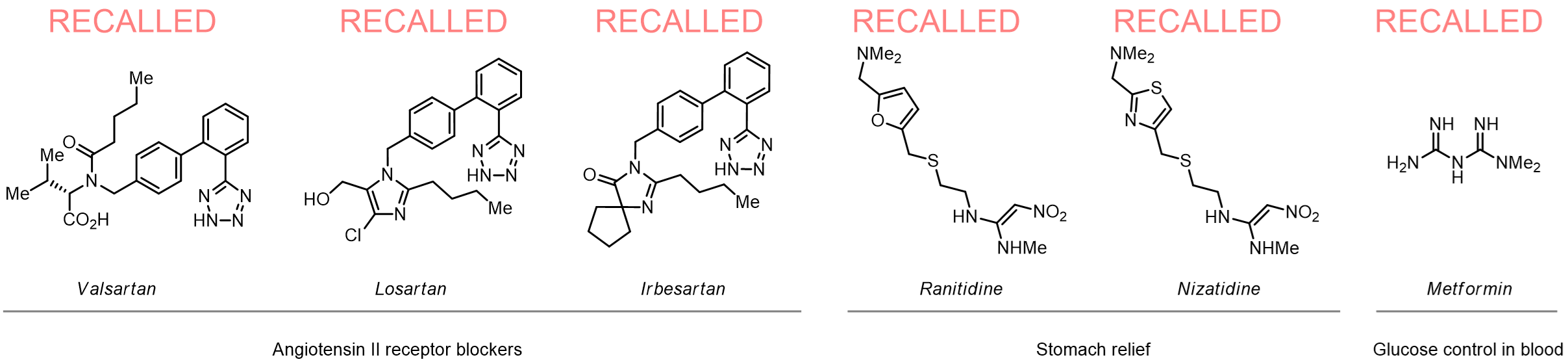
Since 2018, several drugs have been recalled from the market following the discovery of N-nitrosamine impurities in their commercial batches. The N-nitrosamines have been linked to an elevated risk of several cancers especially in case of continuous intake. Since 2020, the FDA and other authorities worldwide have mandated control of N-nitrosamine levels in commercial drugs.
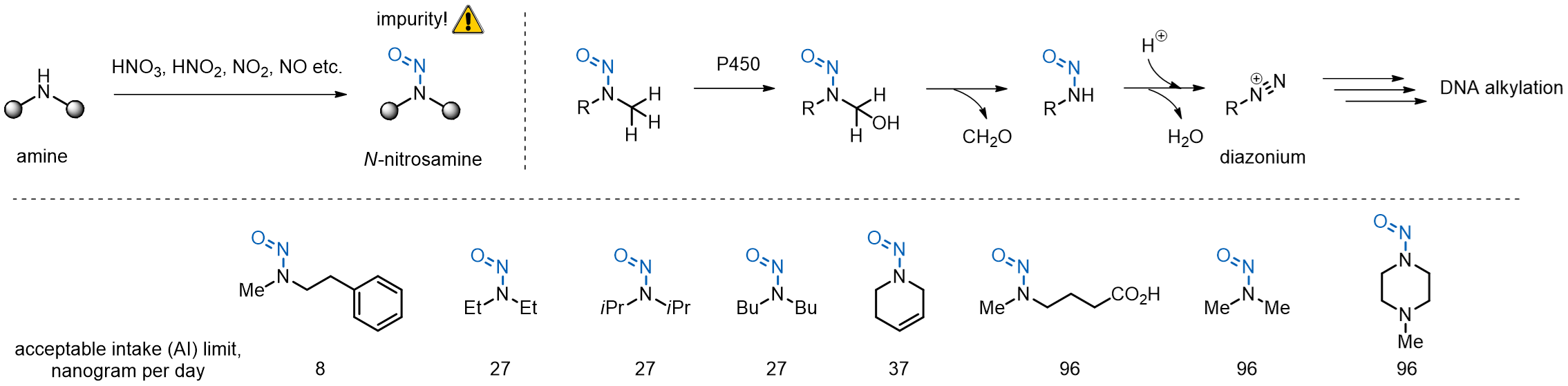
Mechanism
N-nitrosamines can form as impurities in the process of chemical drug production. Following the drug administration, oxidation of the N-nitrosamine species by cytochrome P450 leads to downstream production of cancerogenic diazonium salts that damage DNA by alkylation of susceptible nucleobases. Acceptable daily intake limits have been mandated by authorities for a large panel of N-nitrosamine species, which is, however, not explicit.
Download SD file
Download PDF file
We offer
More than 200 N-nitrosamines from stock on a 5-10 g scale.
Most diverse linkers for your discovery program
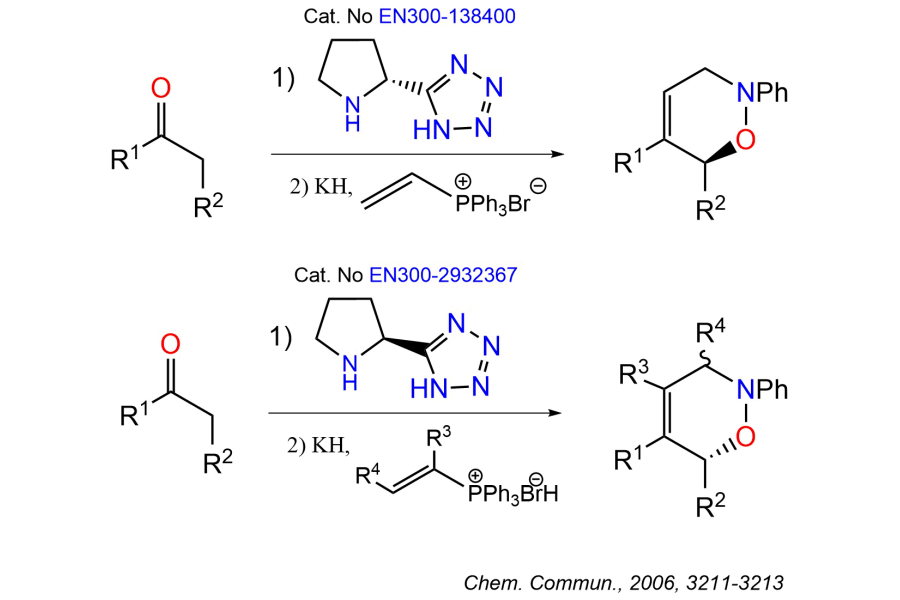
Bifunctional Molecules such as PROTACs, DUBTACs, LYTAC, AUTOTAC, GlueTAC, CHAMP, and others are among the hottest new modalities in modern Drug Discovery. These molecules can be considered as two actives connected with linkers. Numerous scientific studies suggest that the linker’s length, polarity, and rigidity can play a crucial role in a tertiary complex formation, its stability, and the pharmacokinetic (PK) properties of the bifunctional molecules. Quick and easy access to various linkers is essential for a rapid “Linkerology” search since it is next to impossible to predict a “perfect linker” for a given target. Vast majority of protein degraders were developed through the most empirical optimization of linker composition, often driven by the commercial availability of PEG- and carba-precursors.2 As Derek Lowe mentioned in his post on Linkerology: “The only thing we can say for sure now, is that it's a complex situation with a lot of room for surprises. Go make some more linkers!”
Being the largest supplier of Building Blocks, Enamine provides a wide assortment of linker molecules from stock. They can be used for the rapid design and synthesis of different bifunctional molecules. Fine adjustments can also be made by searching over 5 million MADE Building Block linkers.
Download SD files
Linear linkers
These are classical tools that are often applied for the design and investigation of new PROTACs, DUBTAGs, and other bifunctional molecules. Enamine offers a diverse collection of building blocks for the construction of linear PEG- and carba-linkers with different lengths, tail functional groups (FGs), and composition. This collection is continuously enriched with new compounds from our synthesis program.
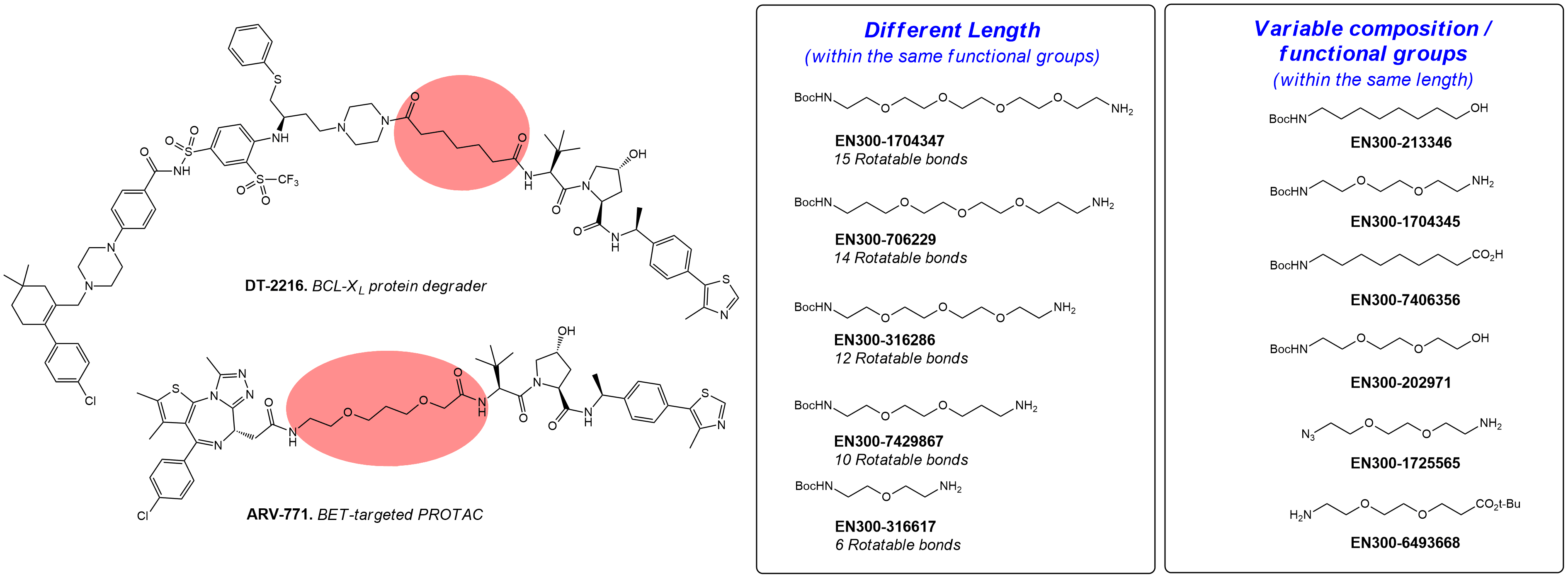
Examples of PROTACs with linear linkers.
Advanced linkers
More complex, with strained structure linkers have become popular in the last couple of years. These advanced linkers contain in their structure cycles, spirocycles, double and triple bonds, and multiple heteroatoms.
Based on the literature data, we singled out the following general criteria for this linkers type: 1-3 cycles, maximum one aromatic/heteroaromatic ring, presence of end functional groups (with or without protection), absence of side functional/reactive group/motifs. The corresponding linker Building Blocks are available from our stock. We can also readily synthesize any other linker molecules upon your request.
Mono-Boc diamines
Mono-Boc diamines are the most popular linkers in the design of bifunctional molecules (first of all PROTACs). Various diamine linkers can be found in the structures of the drug candidates such as ARV-110, ARV-471, CFT8634, and FHD‑609. Recently Janssen chemists have demonstrated a straightforward Direct-to-Biology (D2B) approach to new protein degrader discovery starting from diverse N-Boc diamines.1 Enamine provides an outstanding collection of diverse diamines (by flexibility/distance/shape/polarity) which are useful for linkerology studies of new bifunctional molecules.
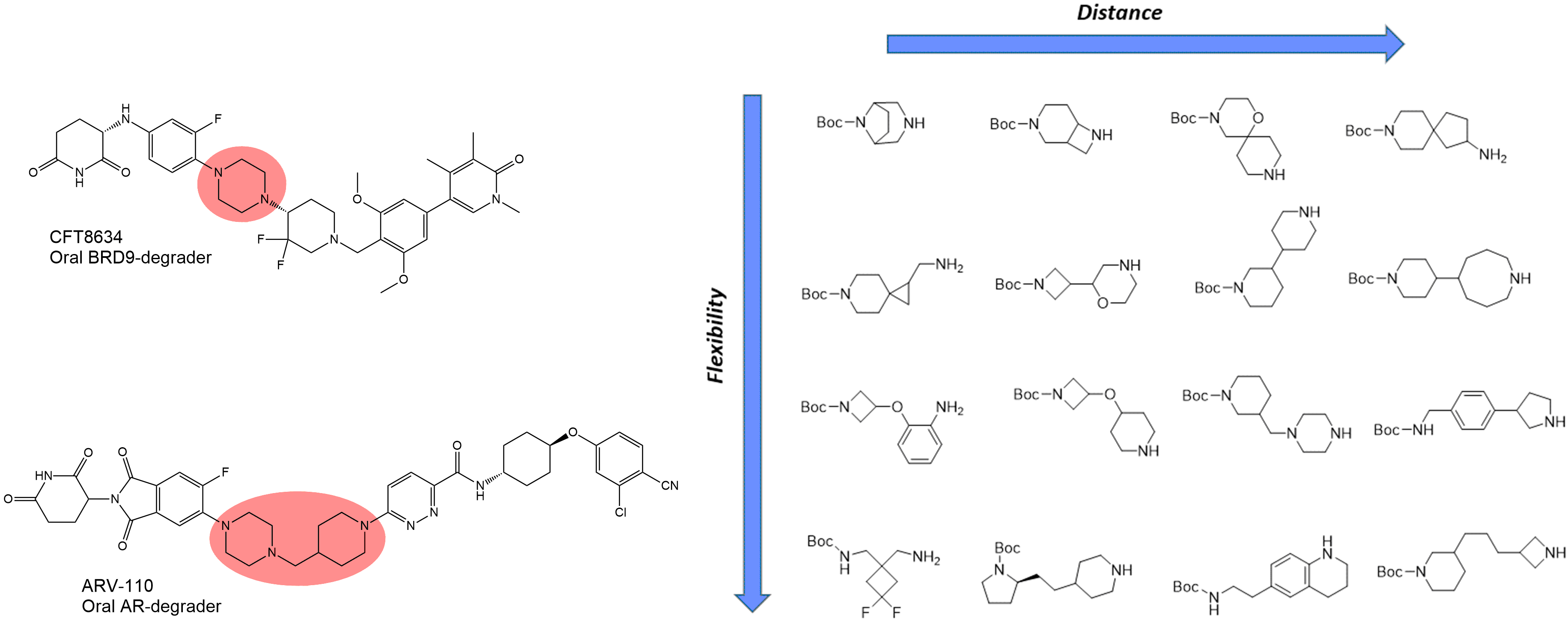
Other linkers
Besides diamines other linkers with diverse end-functional groups are widely used in the design of bifunctional molecules. Enamine provides various linkers with different combinations of functional groups (e.g., OH/halogen-acetylenes, amino acids, aminoacetylenes, aminoazides). Upon request, we can propose a set of linker Building Blocks according to your criteria.
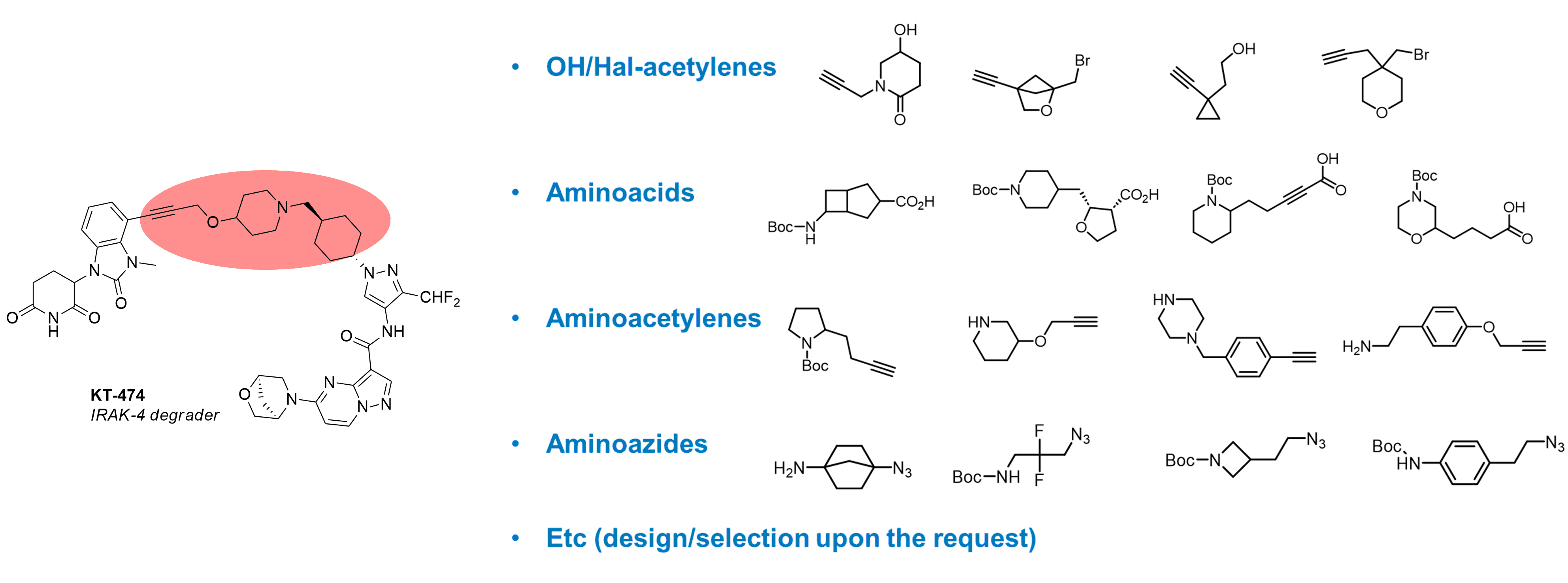
Selected publications
-
Direct-to-Biology Accelerates PROTAC Synthesis and the Evaluation of Linker Effects on Permeability and Degradation.
ACS Med. Chem. Lett. 2022, 13, 7, 1182–1190. DOI: 10.1021/acsmedchemlett.2c00124 -
Current strategies for the design of PROTAC linkers: a critical review.
Explor. Target Antitumor Ther. 2020, 273-312. DOI: 10.37349/etat.2020.00018