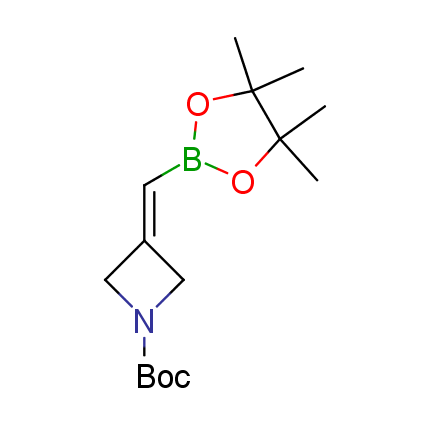
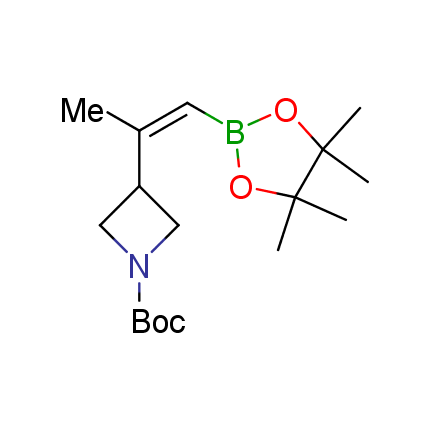
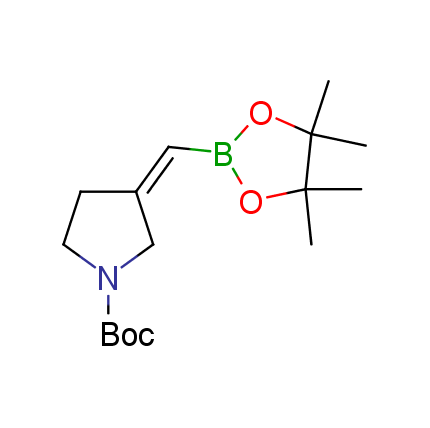
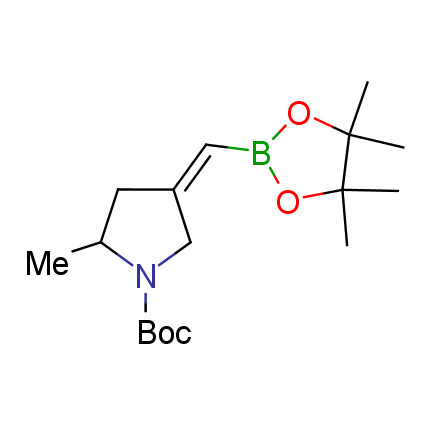
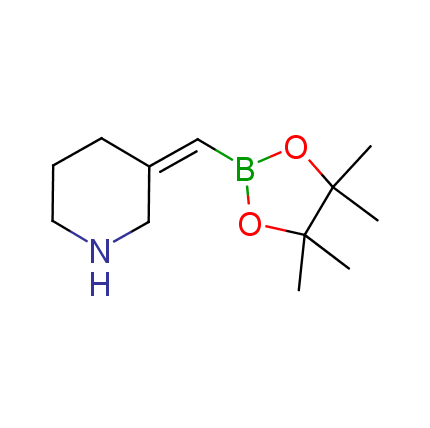
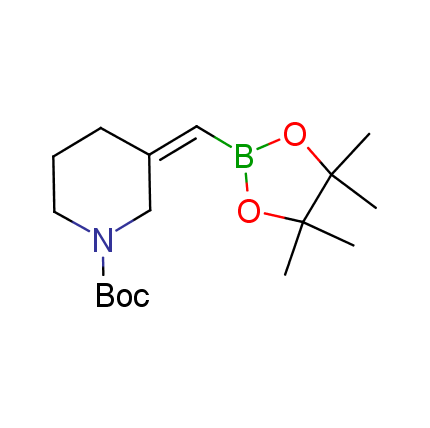
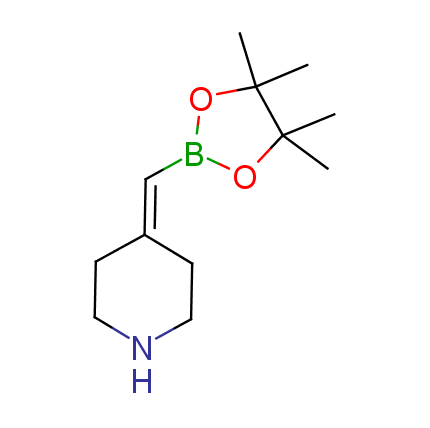
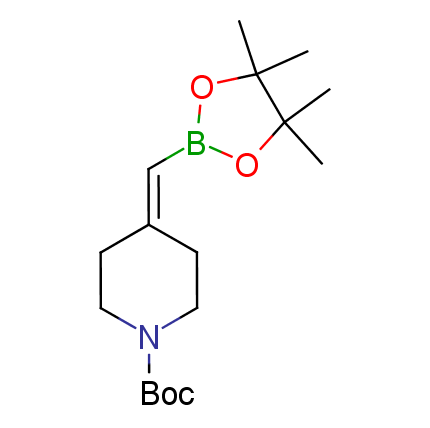
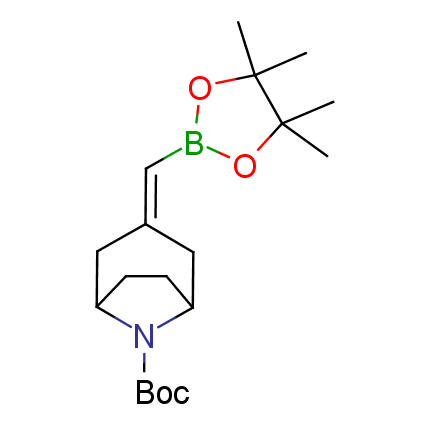
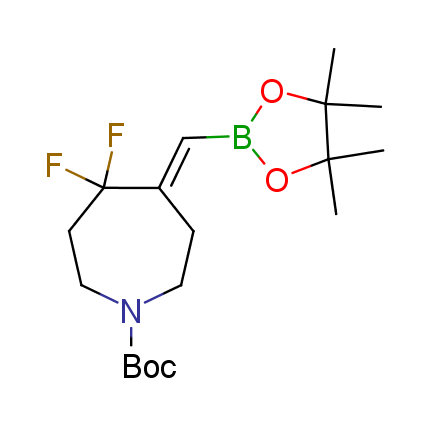
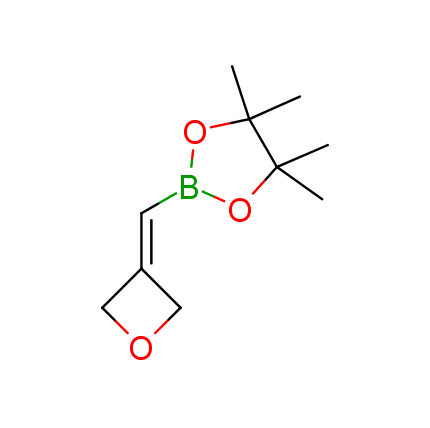
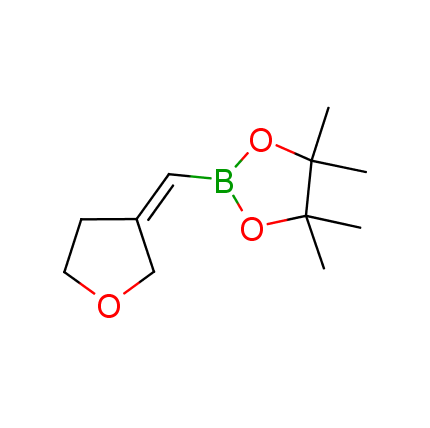
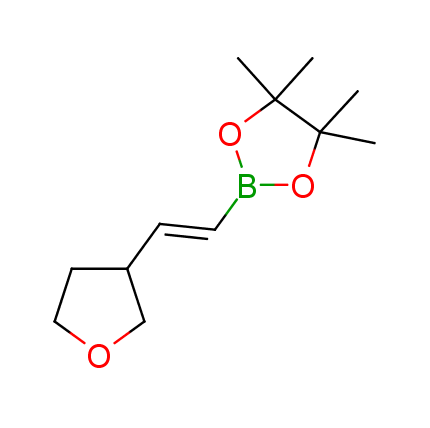
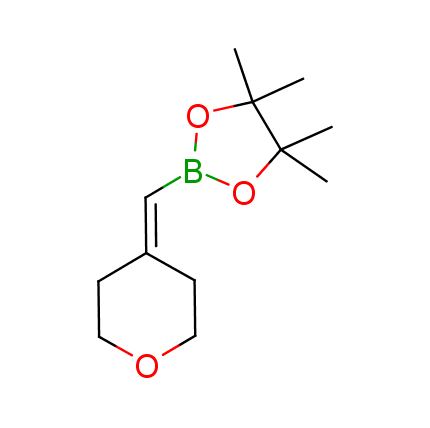
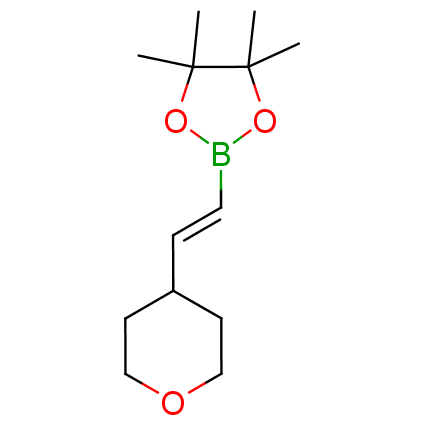
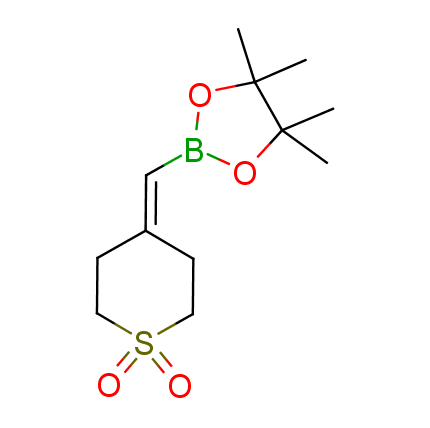
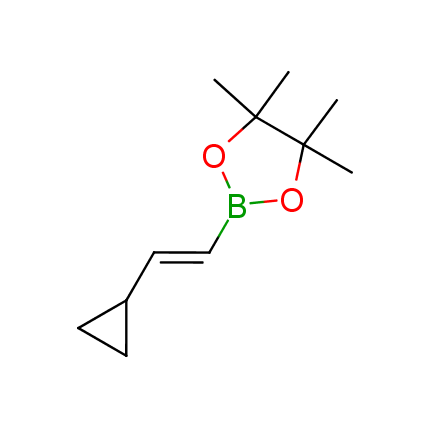
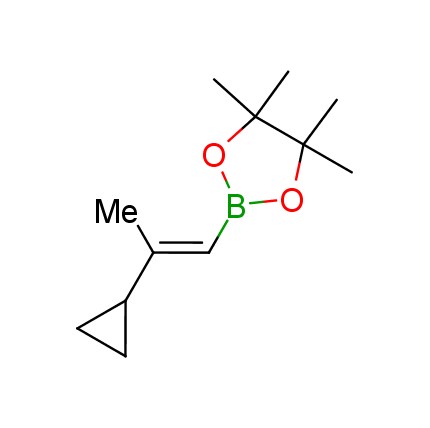
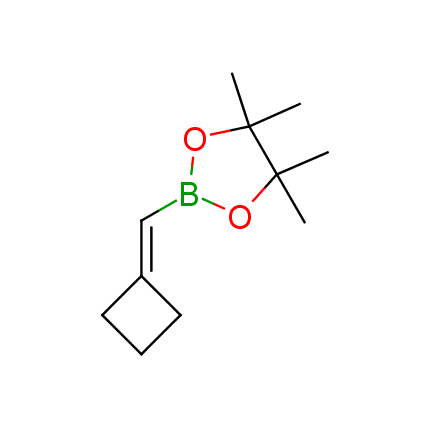
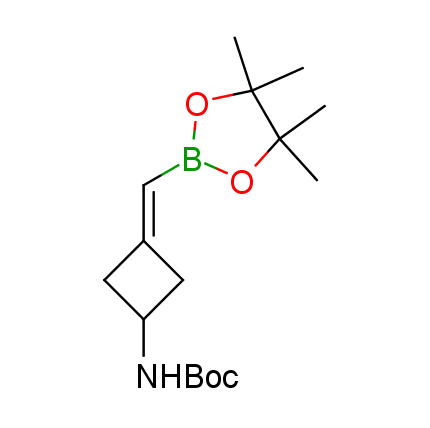
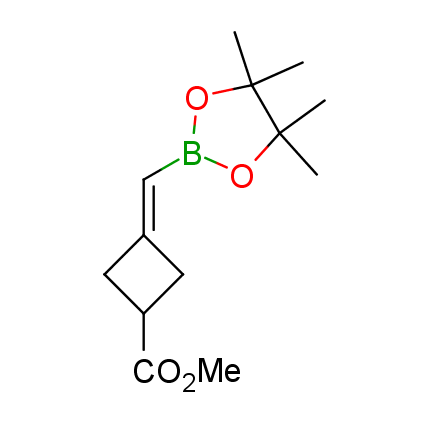
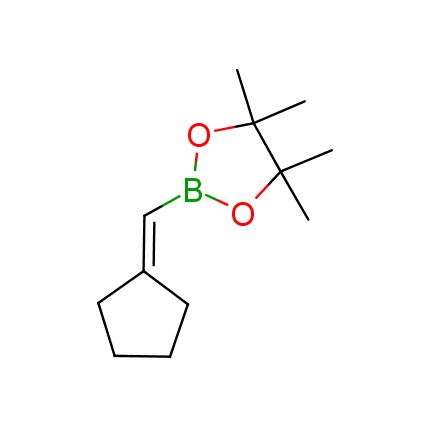
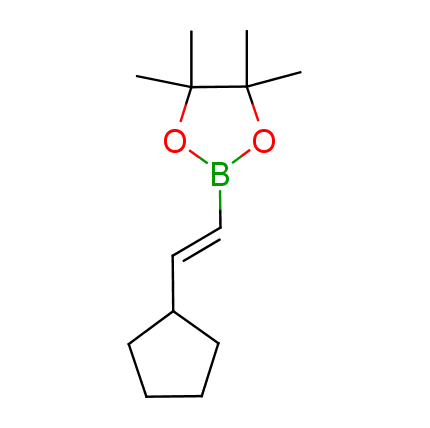
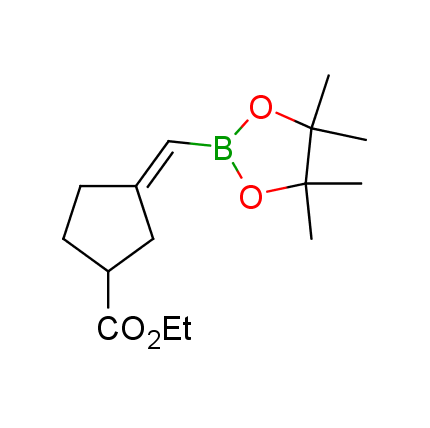
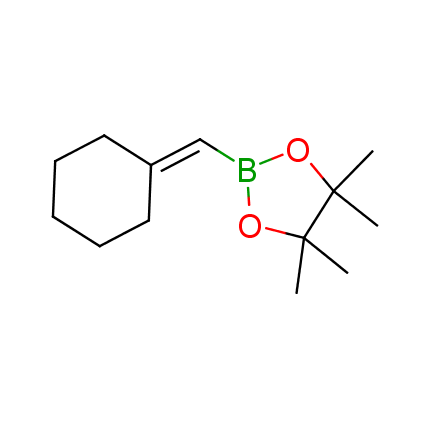
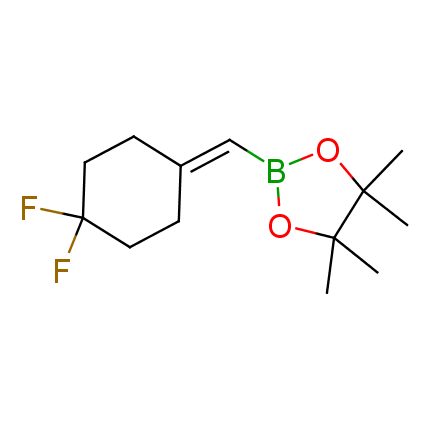
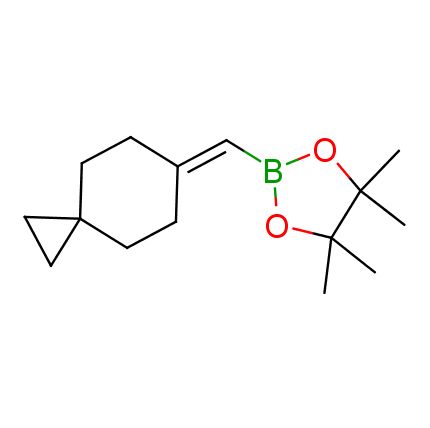
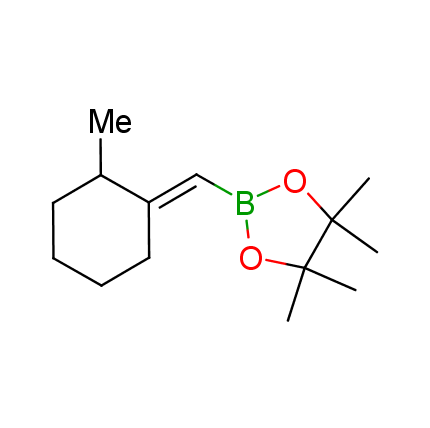
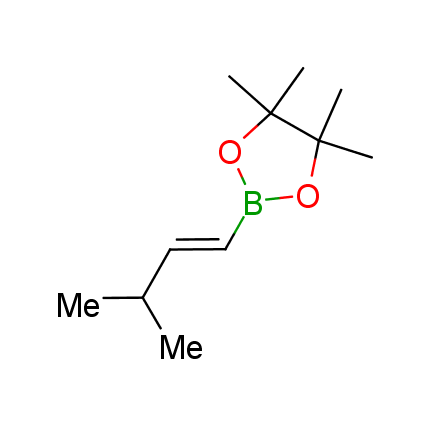
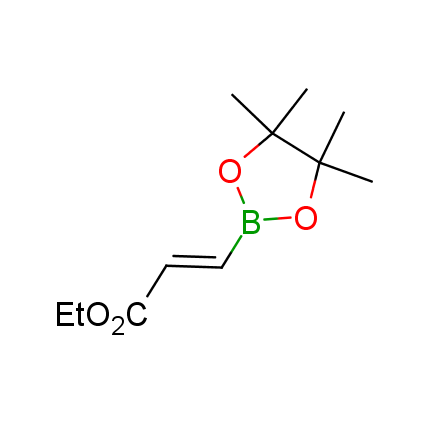
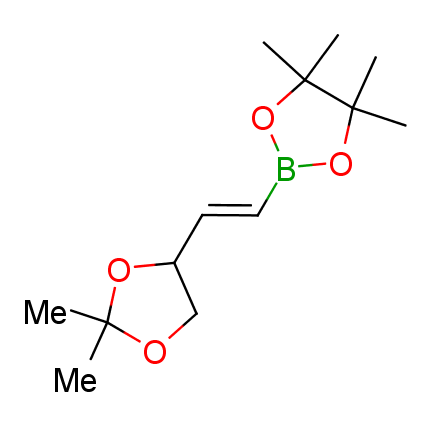
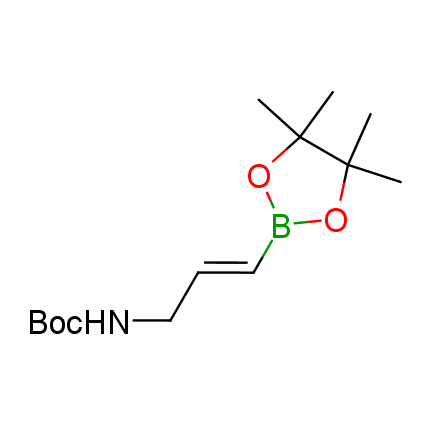
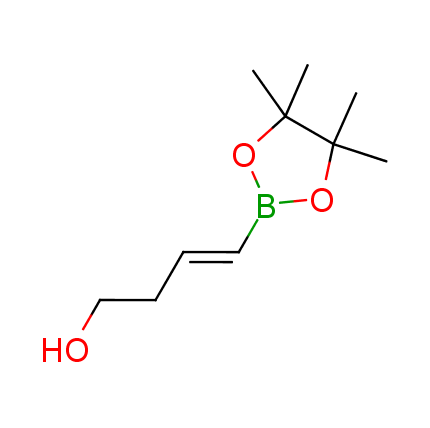
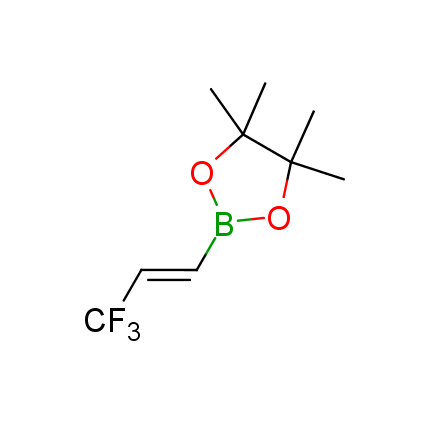
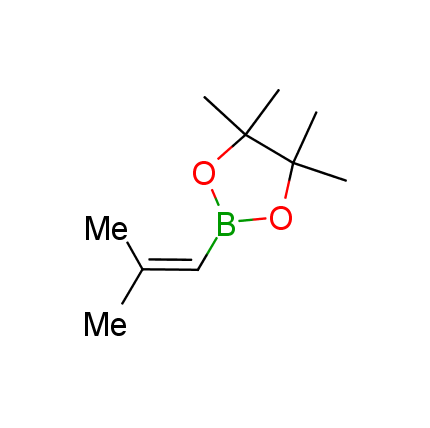
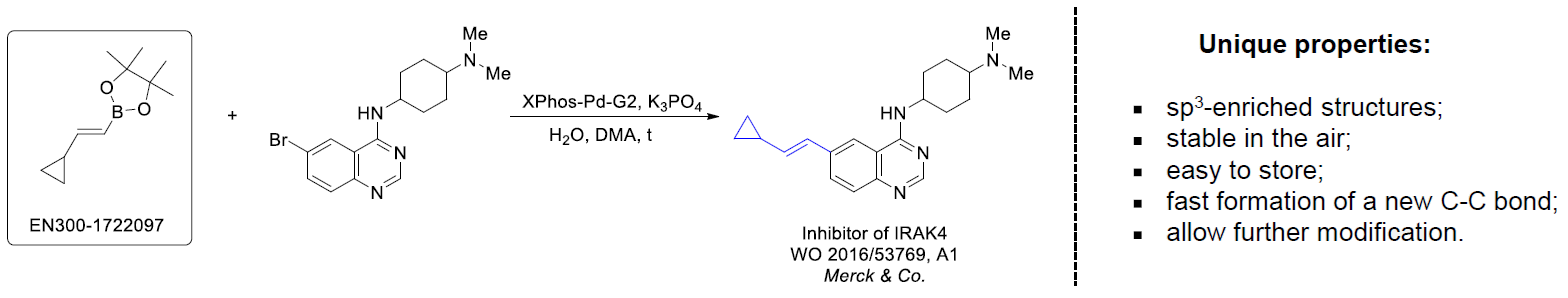
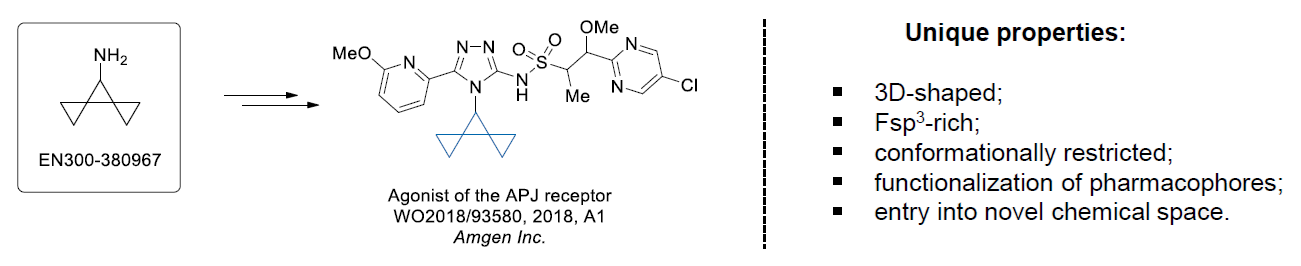
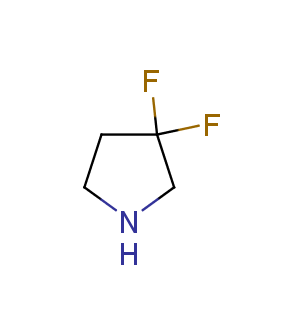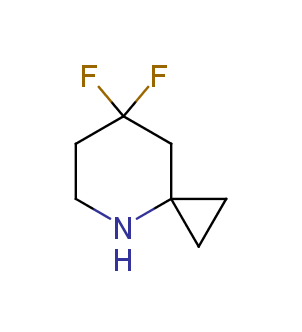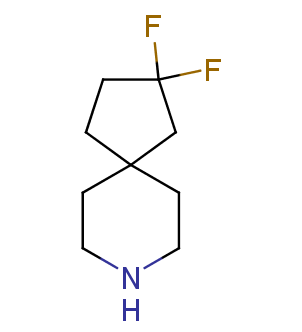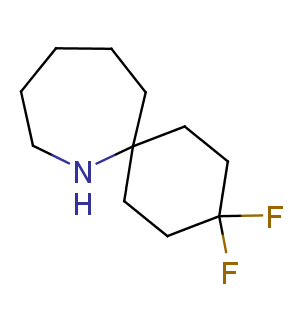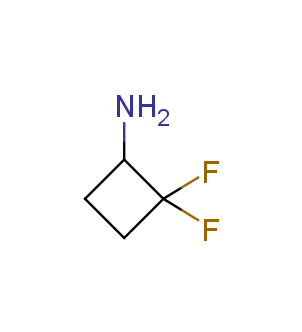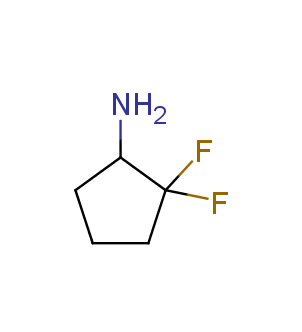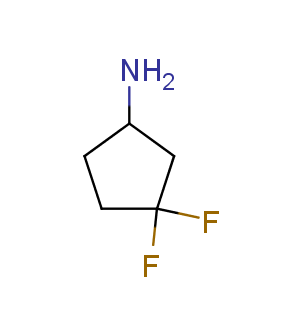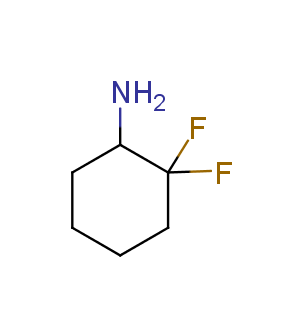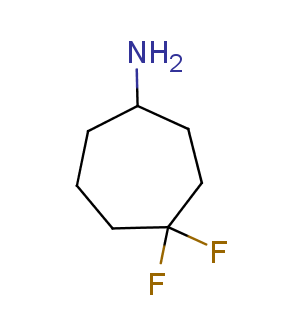The Suzuki–Miyaura cross-coupling of boronic acid derivatives is one of the most used reactions in organic and medicinal chemist's toolbox. The rapid advancement of this method resulted in its efficient application for the late-stage modification of biologically active substrates and construction of combinatorial libraries. One of the recent trends in the field of organoboron reagents is related to the shift from aromatic compounds towards cyclic sp3-enriched structures, which comply with criteria of lead-oriented synthesis. In this context, Enamine offers a library of alkenylboronic esters for metal-mediated couplings.
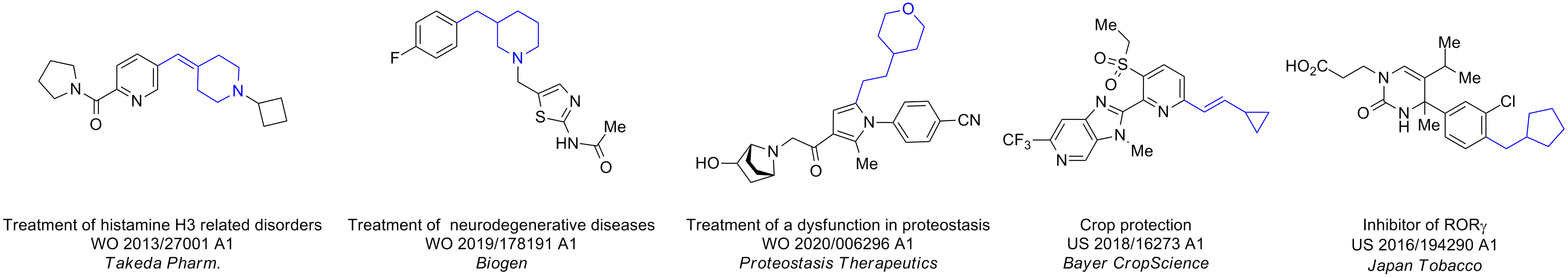
Case studies
Download SD file
Download PDF file
We offer:
more than 50 of vinyl boronates from stock on a 5-10 g scale.
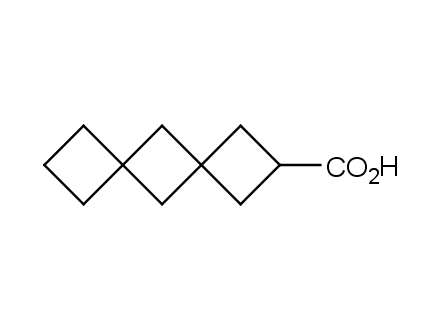
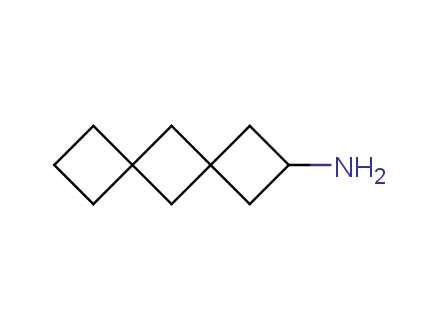
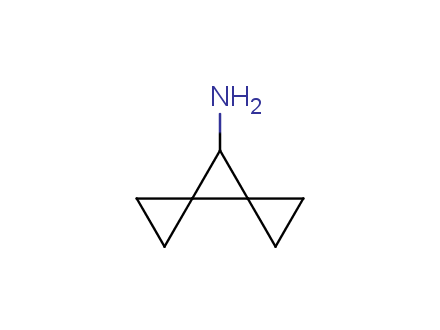
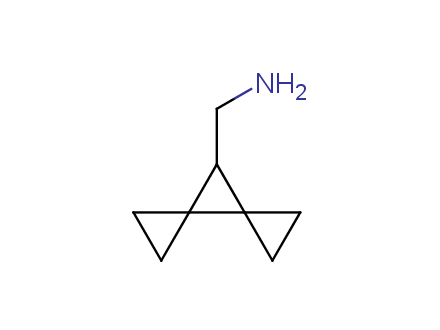
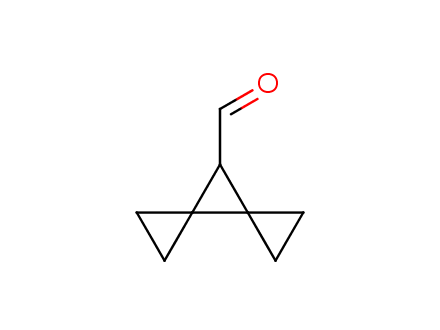
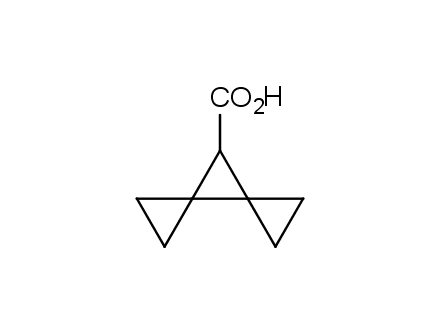
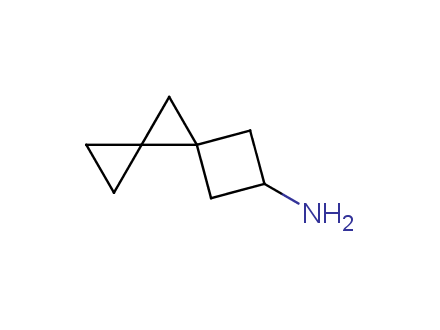
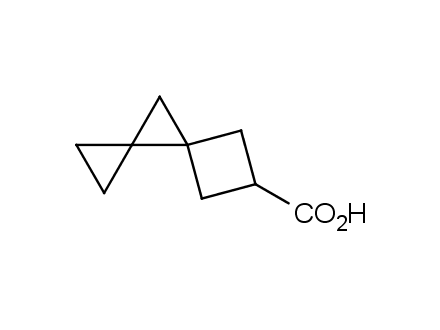
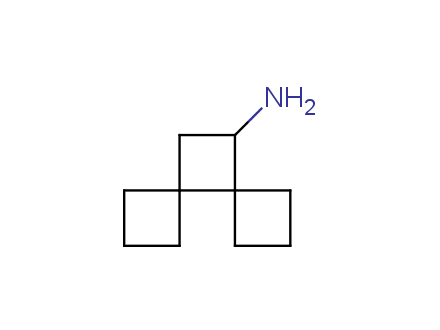
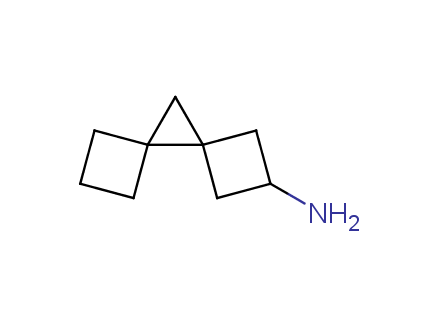
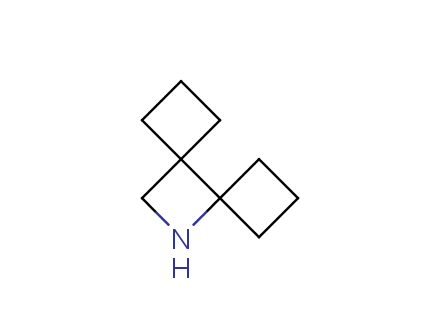
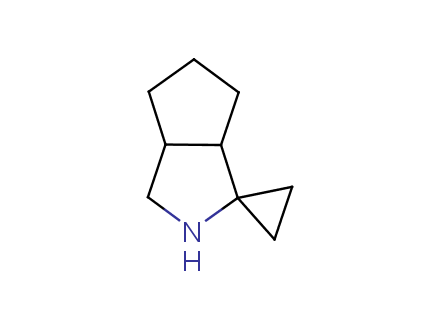
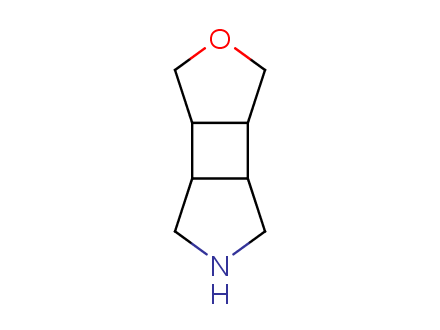
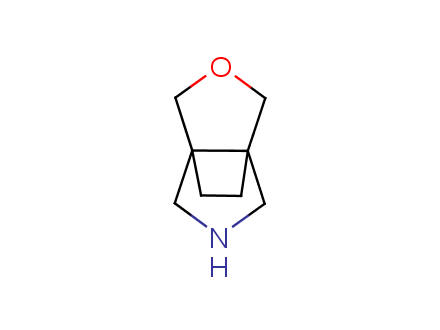
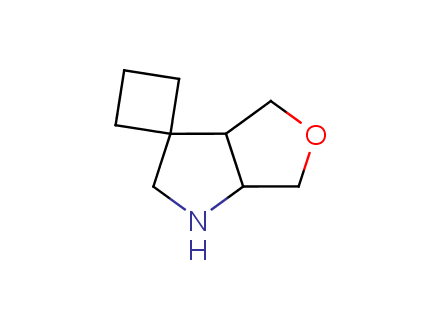
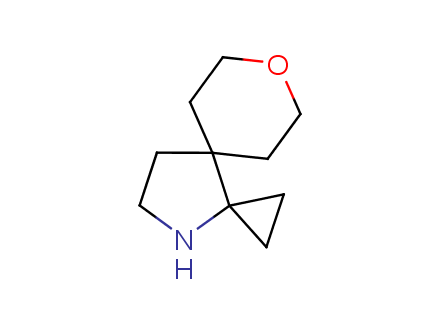
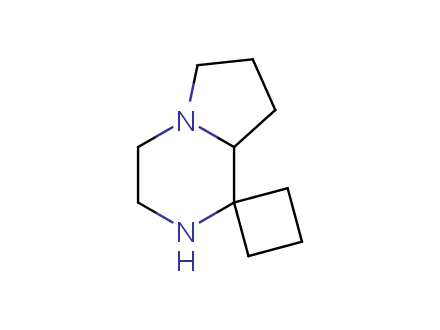
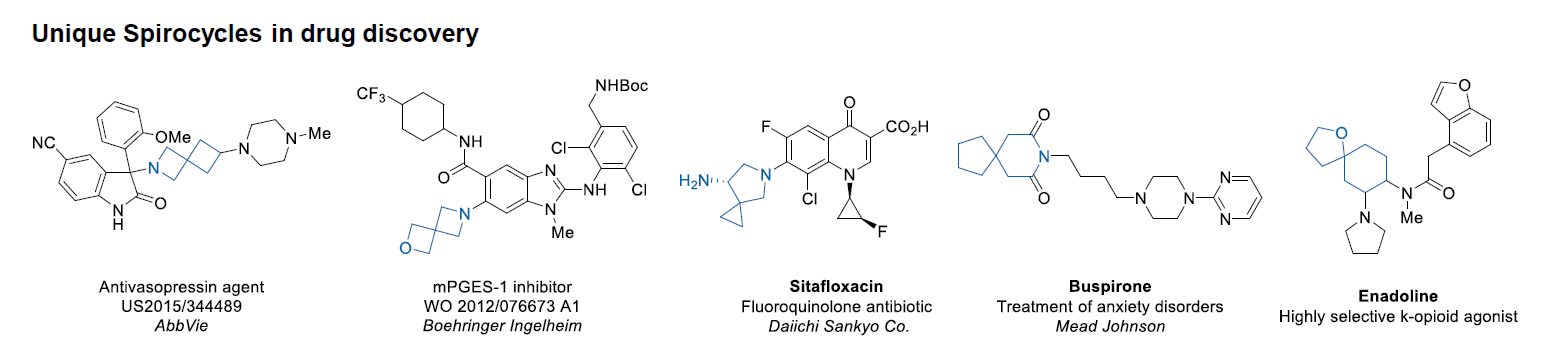
Conformational rigidification of flexible compounds by introducing a ring is a popular strategy in drug design. The resulting cyclic analogues usually have a reduced conformational entropy penalty upon binding to a protein target. A conformational restriction can also be imposed by introduction of a spirocyclic ring. Spirocyclic systems are 3D-shaped, in strict contrast to flatten benzene compounds. It is especially true for polycyclic compounds. In this context, Enamine offers a library of innovative three-cyclic scaffolds for drug design.
Case studies
Download SD file
Download PDF file
We offer:
More than 50 of three-cyclic building blocks from stock on a 5-10 g scale
The LARGEST catalog in the world
Enamine is a renowned leader in the market of building blocks. Our stock collection counts 300 000 building blocks on a gram scale, most of which being unique – not available from other suppliers. These compounds have been synthesized in-house and in collaboration with FCH Group, Intermed Chemicals, and UORSY.
The jointly acquired broad experience now allows us to offer 1.28B MADE (MAke-on-DEmand) Building Blocks. Prices are assigned to each compound proving solid scientific basis and assuring our confidence in their synthesis. It is by far the world’s largest and most comprehensive catalog of all commercially available building blocks with less than 20 effective heavy atoms (not counting protecting groups)! Most of the compounds are novel, not available through other suppliers or other global aggregate platforms. MADE Building Blocks include such popular in medicinal chemistry compound classes as primary and secondary amines, sulfonyl chlorides, boronic acids, carboxylic acids, alkynes, azides, etc.
In the ocean of virtual and me-too compound catalogs, we offer our proven high-end synthesis expertise to deliver our MADE Building Blocks because of:
- Thoroughly documented synthesis protocols (2-4 steps only!) allowing the trouble-free synthesis of next compounds
- In-stock availability of raw materials (incl. ca. 10 000 building blocks in > 1 kg) directly at Enamine enabling immediate synthesis start
Every month, our chemists synthesize around 2 000 new MADE Building Blocks on our customers’ orders. The synthesis typically takes 2-6 weeks and has > 76% success rate.
You can conveniently make an online substructure or similarity search for novel, previously unseen compounds at our partner Chemspace website: https://chem-space.com/search, the only online resource having the comprehensive and most up-to-date collection of MADE Building Blocks.
The MADE Building Blocks are also marketed under brands of FCH Group, and Intermed Chemicals, and UORSY.

Molecular properties

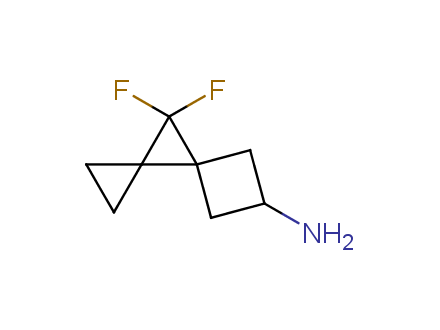
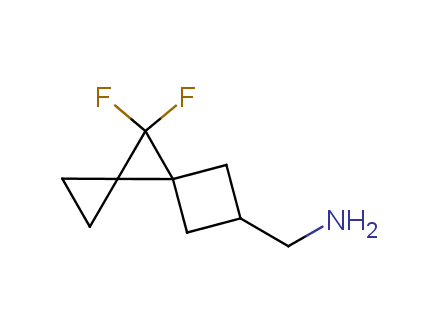
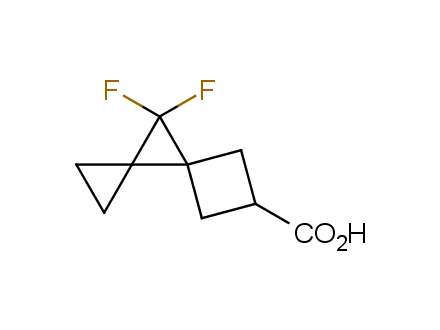
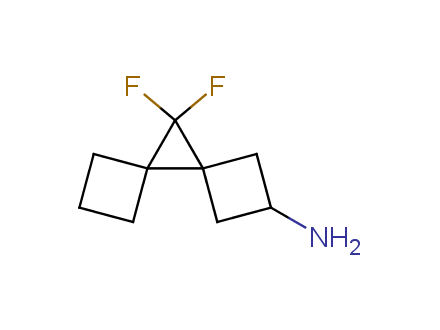
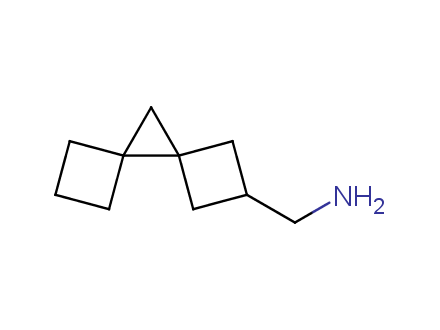
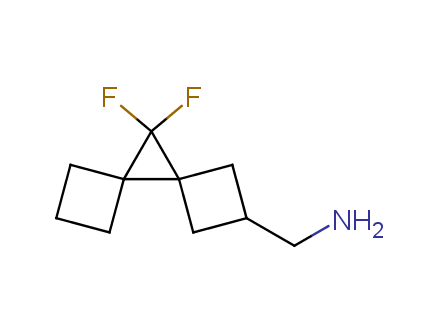
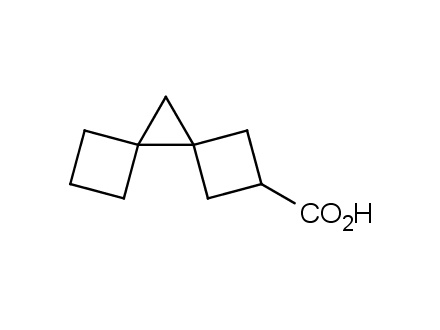
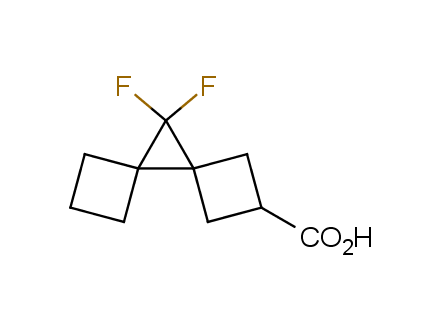
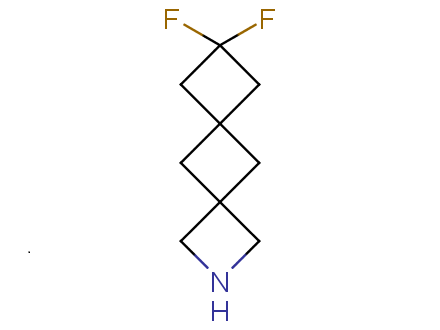
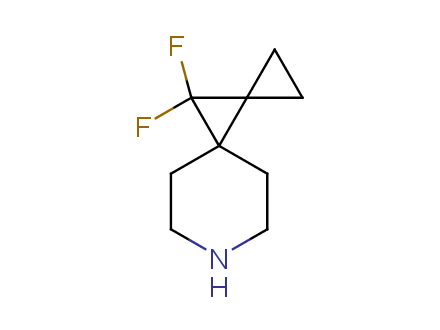
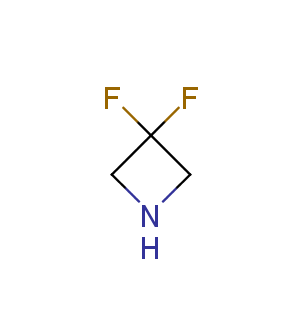
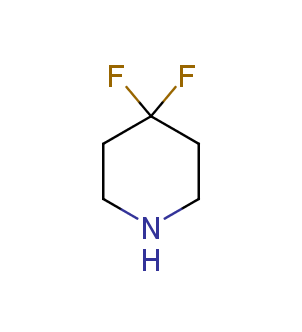
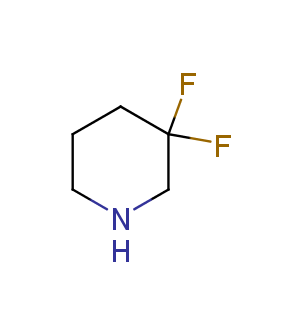
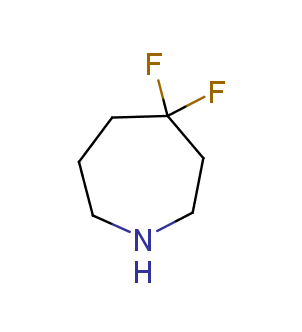
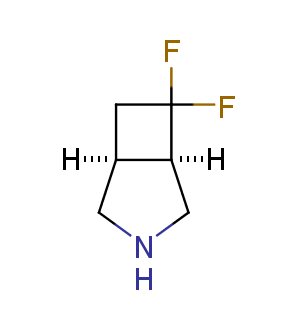
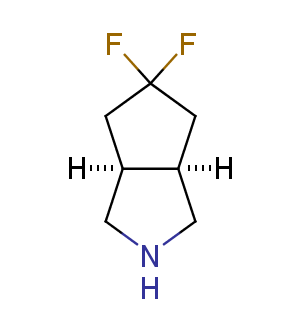
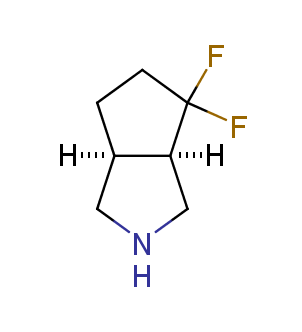
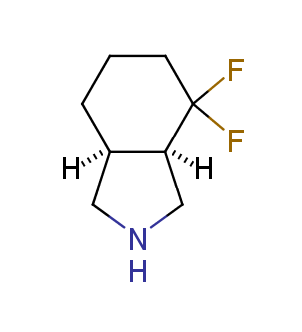
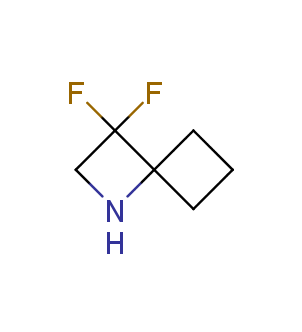
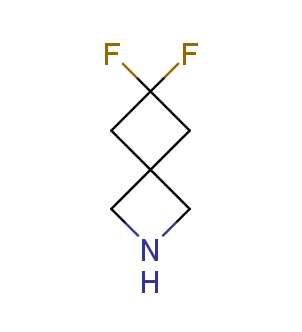
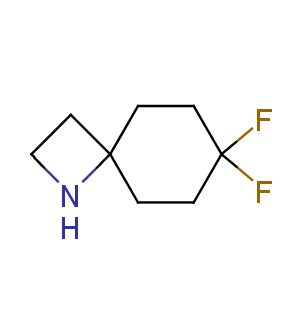
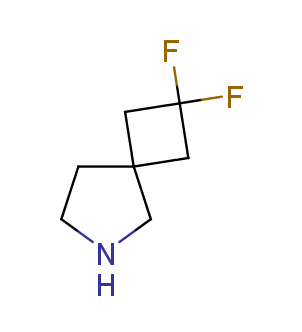
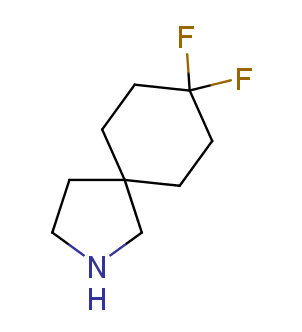
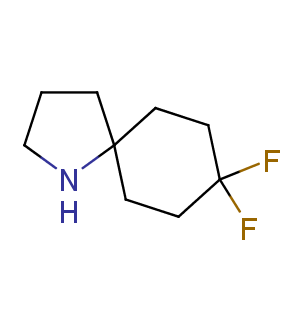
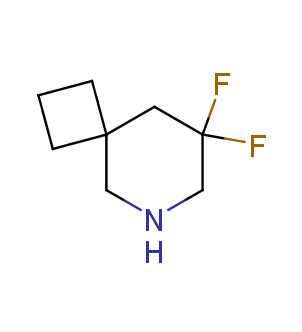
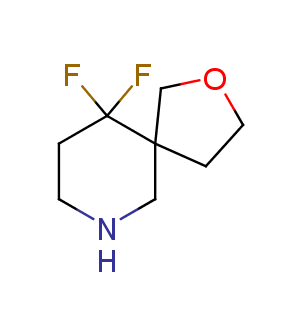
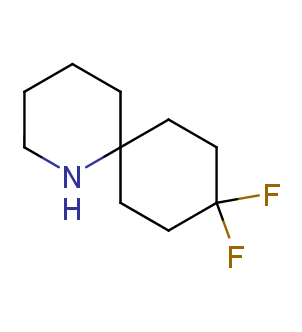
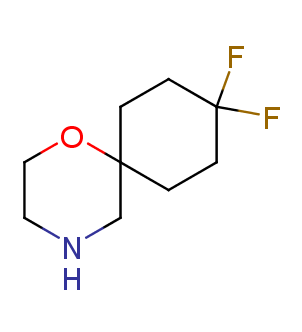
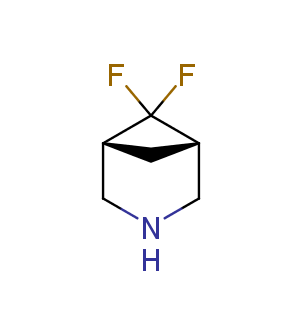
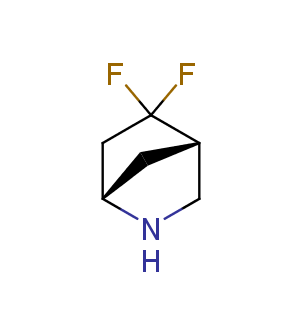
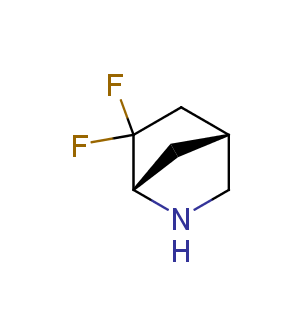
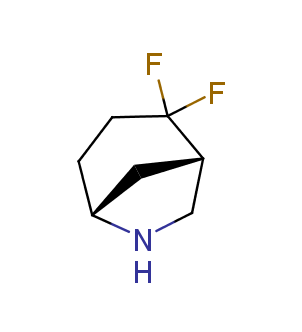
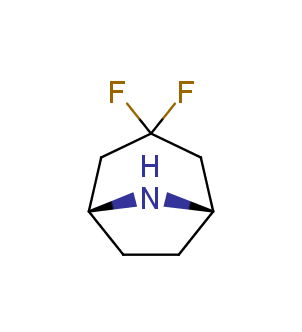
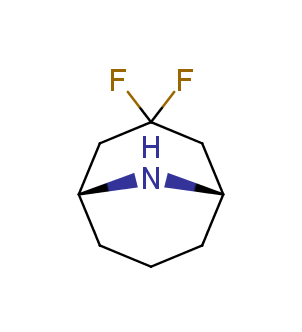
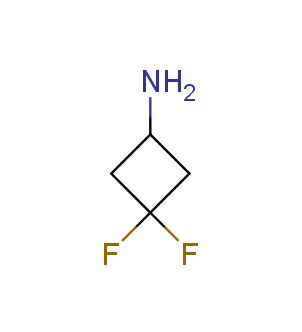
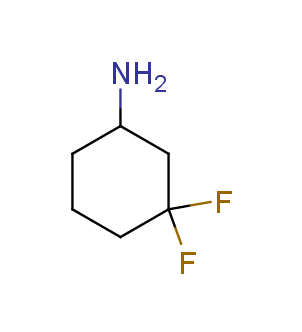
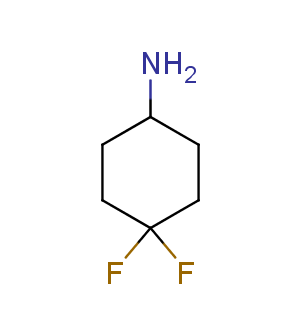
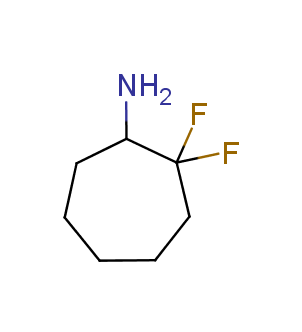
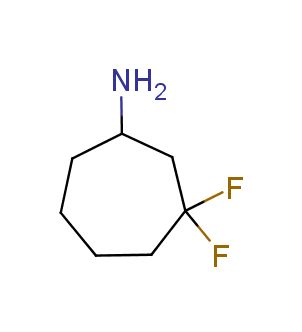
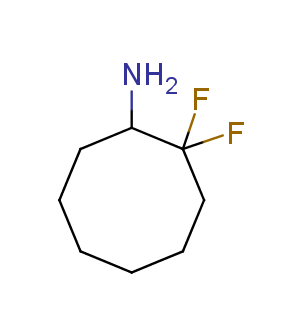
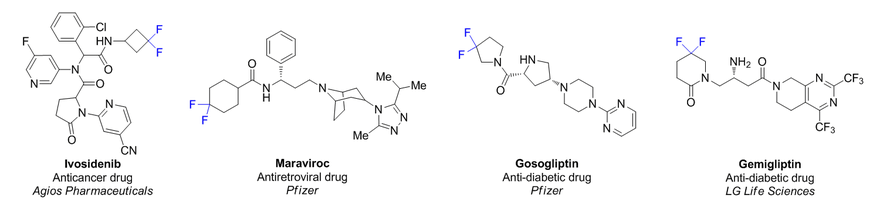
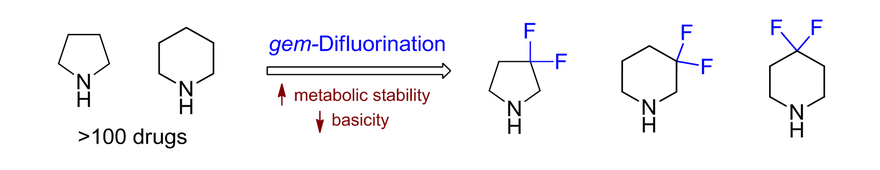
Fluorinated derivatives play an important role in medicinal chemistry. The selective incorporation of a fluoroalkyl group into bioactive compounds often affects their binding affinity, metabolic stability, lipophilicity, membrane permeability and bioactivity. gem-difluoromethylene group (CF2) is a valuable fluorinated motif that is present in pharmaceuticals and biologically active compounds. In particular, gem-CF2-group improves ADME- and PK-properties. In this context, Enamine offers a library of unique difluoro-substituted cyclic amines for drug design.
Concept
Download SD file
Download PDF file
We offer:
>100 gem-difluorinated amines on gram-scale from stock.
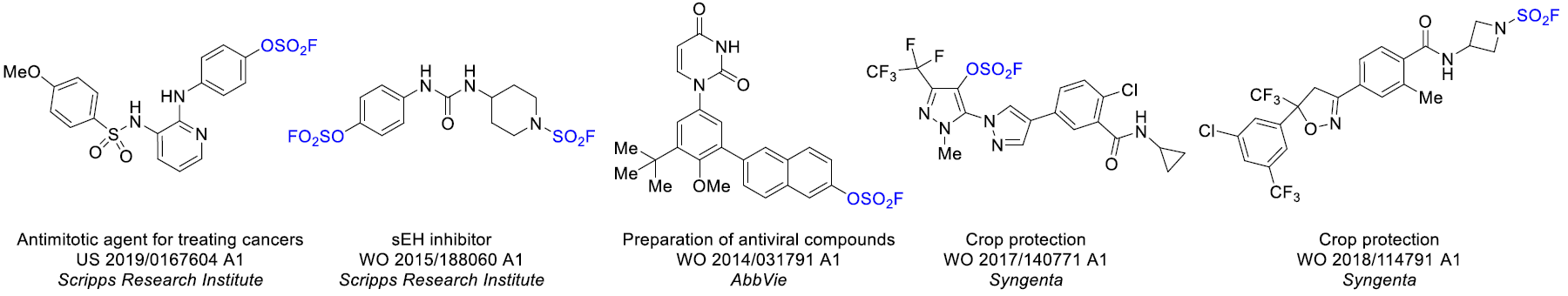
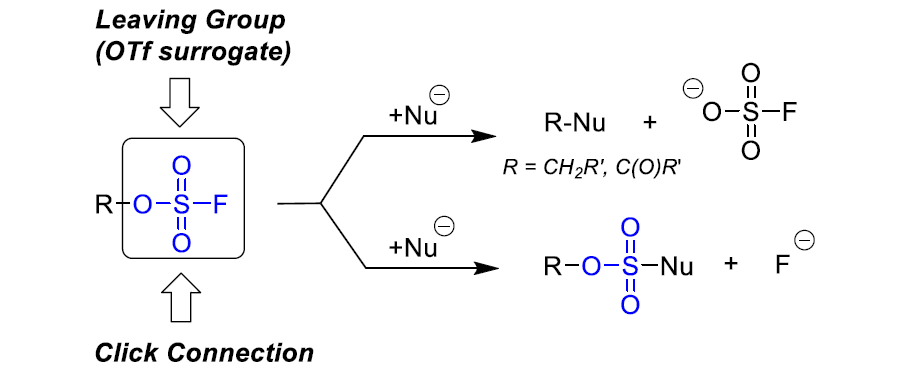
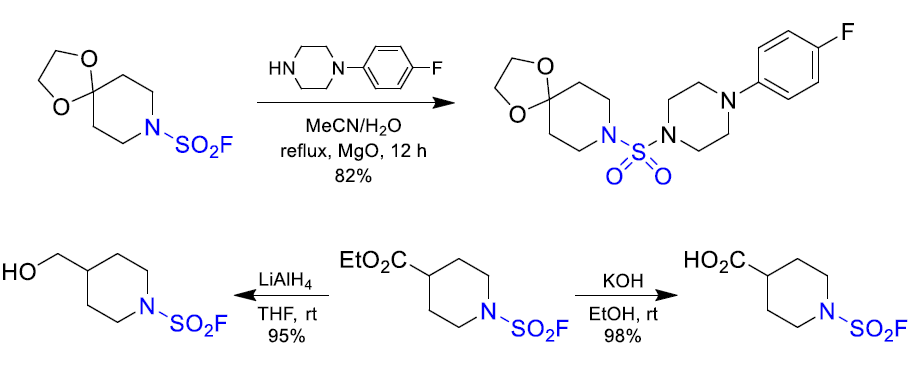
Aryl fluorosulfates and sulfamoyl fluorides are widely used in chemical biology, medicinal chemistry and agrochemistry. The former exhibit chemoselective reactivity with the side chains of tyrosine, lysine, serine and histidine in the proteins, and can be used to target non-enzymes as well as enzymes. Depending on the nature of the substituent, the -OSO2F unit can be a good leaving group or a robust connector. The fluorosulfates are quite stable toward hydrolysis under neutral or acidic conditions. The N-disubstituted sulfamoyl fluorides are stable toward hydrolysis under basic condition, inert toward a wide range of nucleophiles and dramatically more robust than analogous chlorides. In this context, Enamine offers a library of unique aryl fluorosulfates and sulfamoyl fluorides for drug design.
Properties of ROSO2F and R2NSO2F
Dual reactivity of fluorosulfates
Reactivity and stability of the sulfamoyl fluorides
Download SD file
Download PDF file
We offer:
>100 unique fluorosulfates and sulfamoyl fluorides in gram amounts in stock.