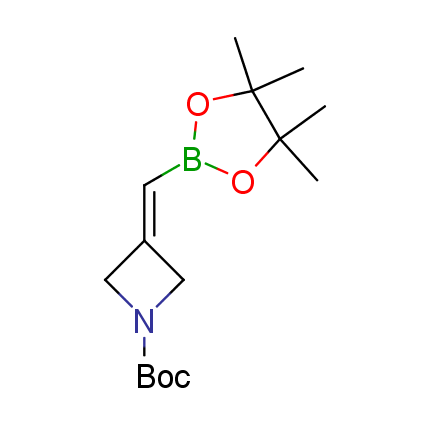
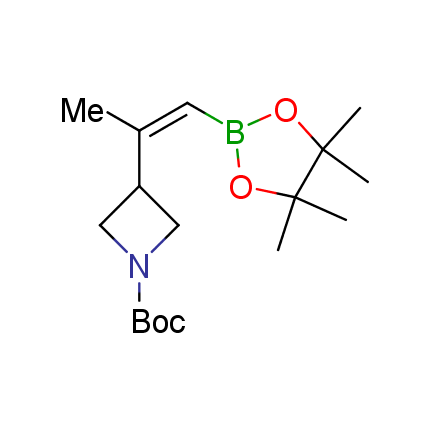
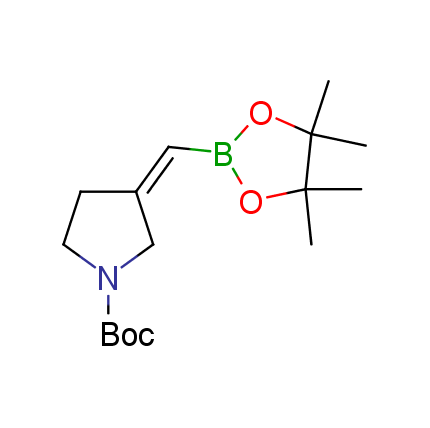
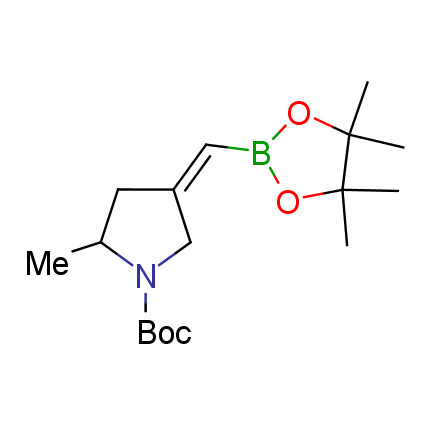
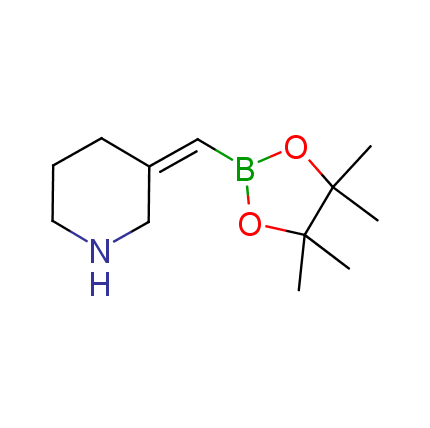
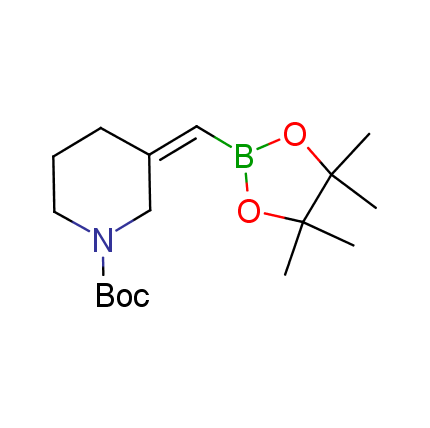
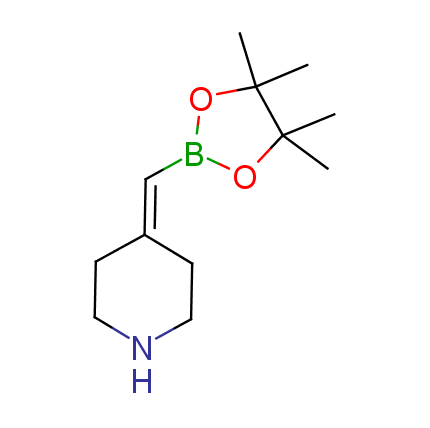
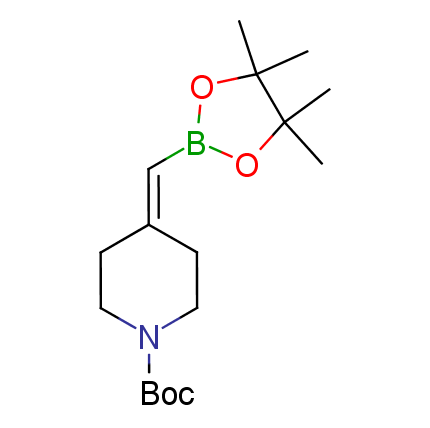
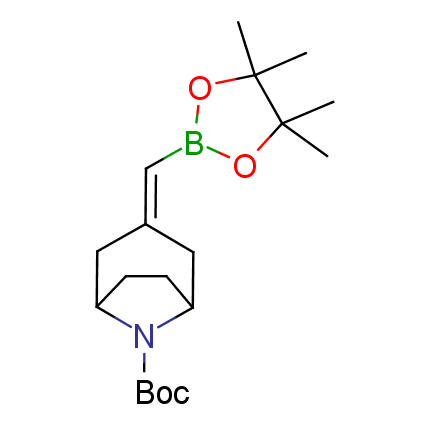
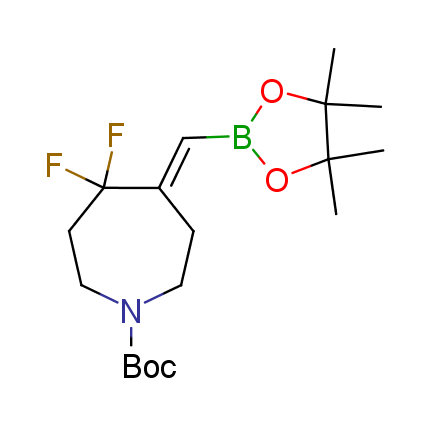
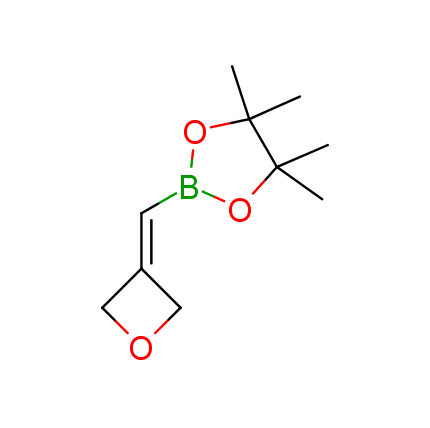
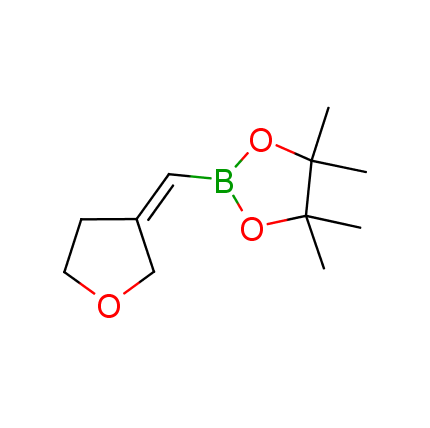
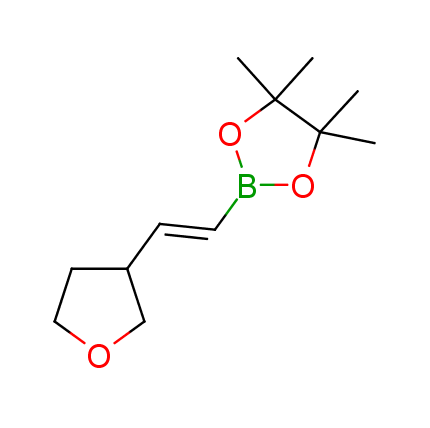
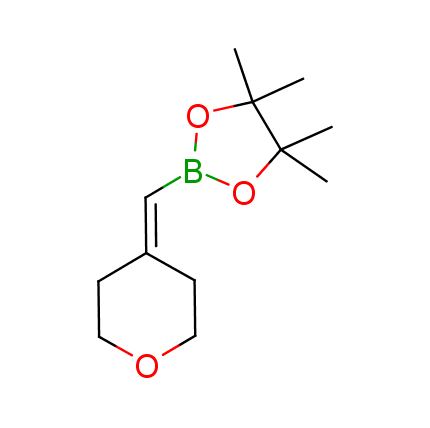
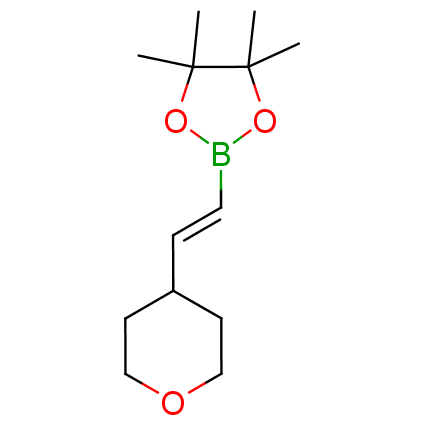
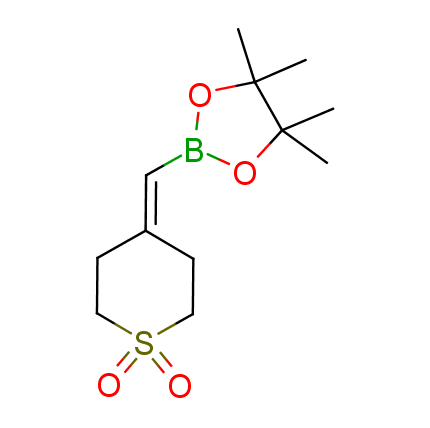
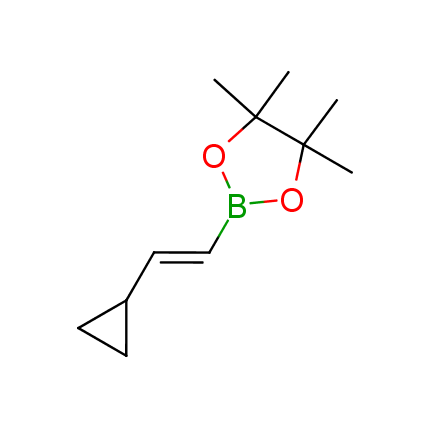
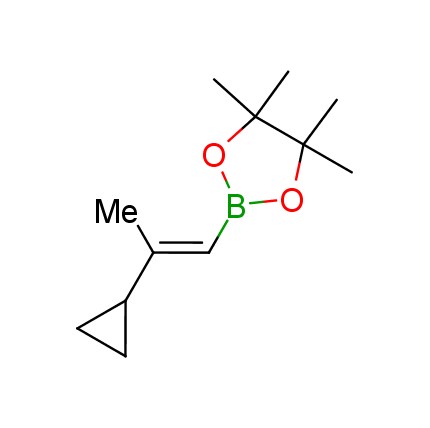
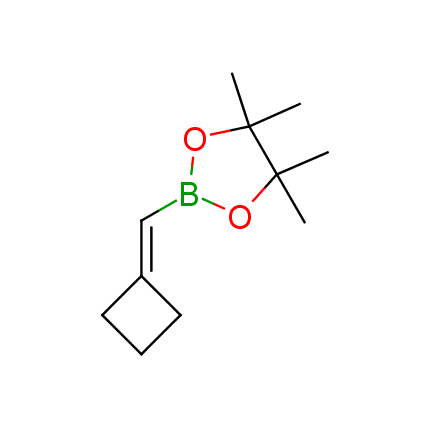
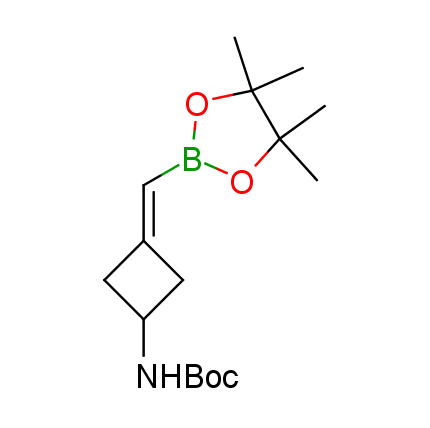
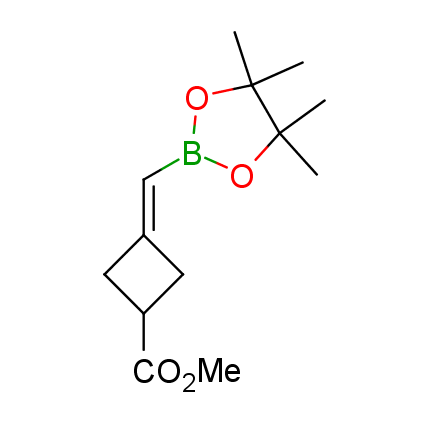
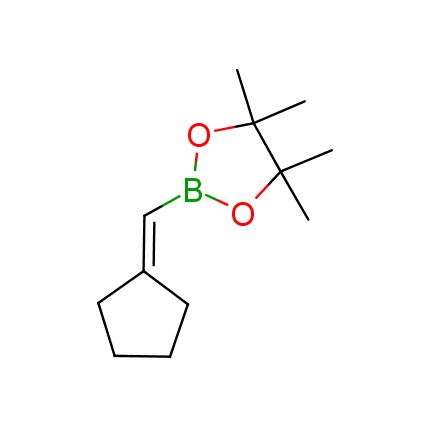
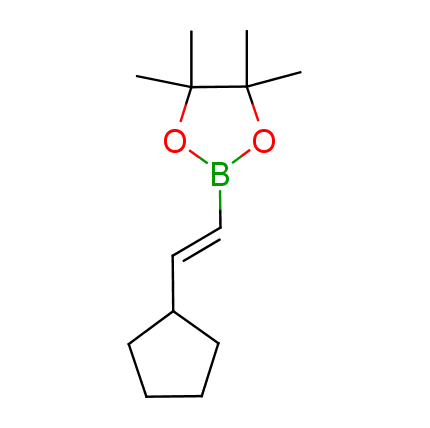
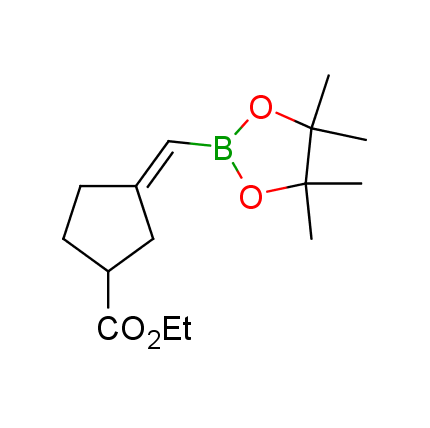
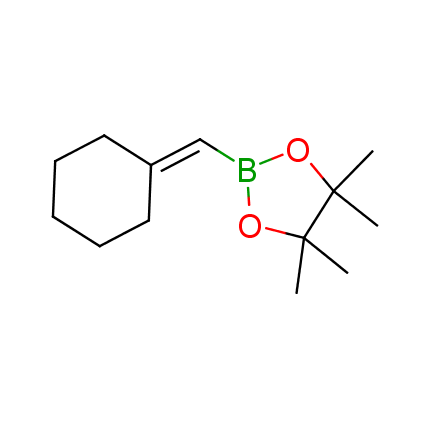
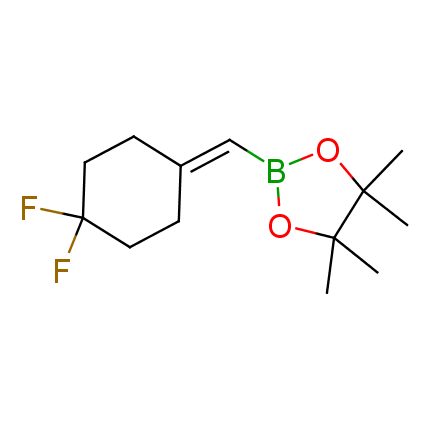
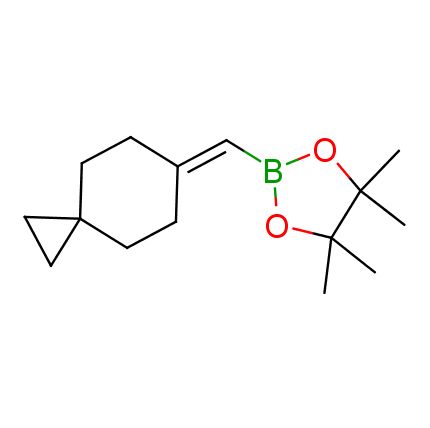
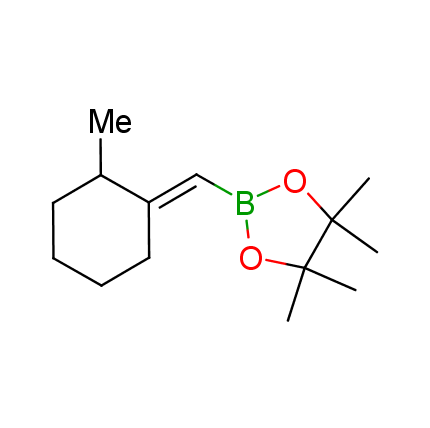
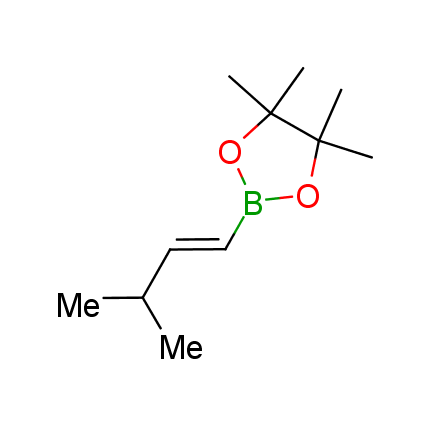
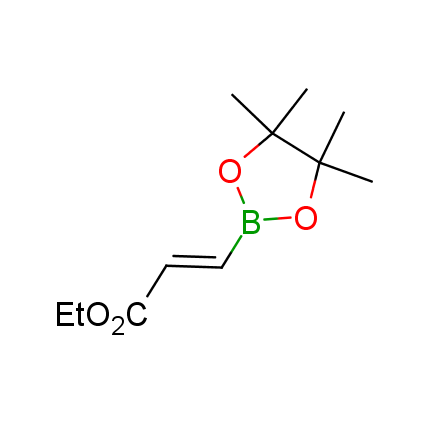
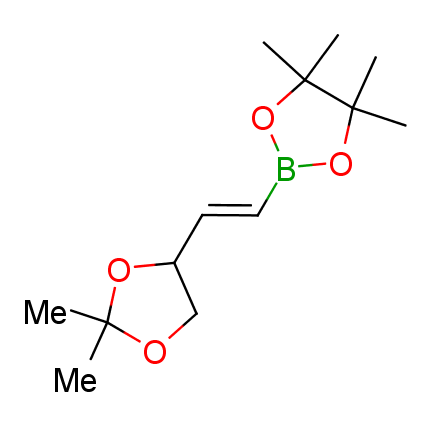
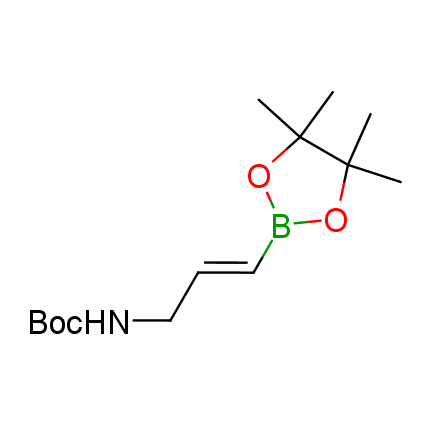
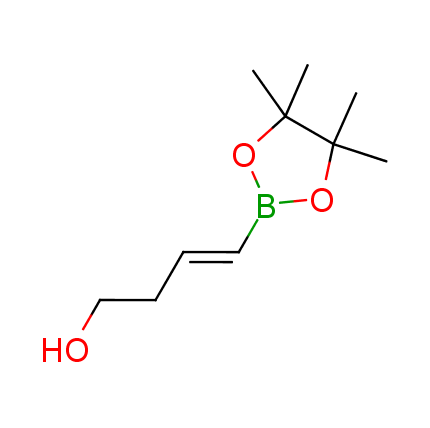
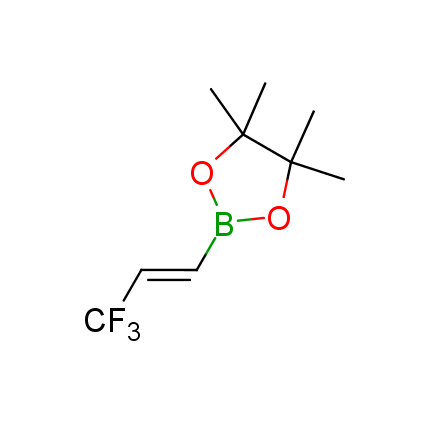
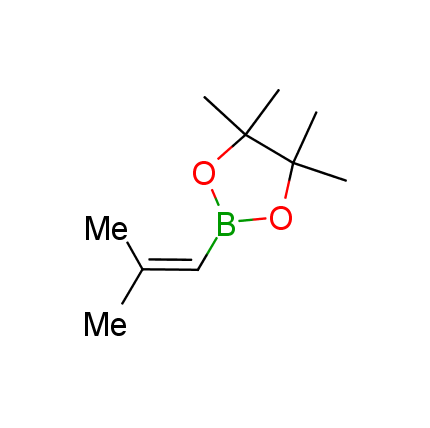
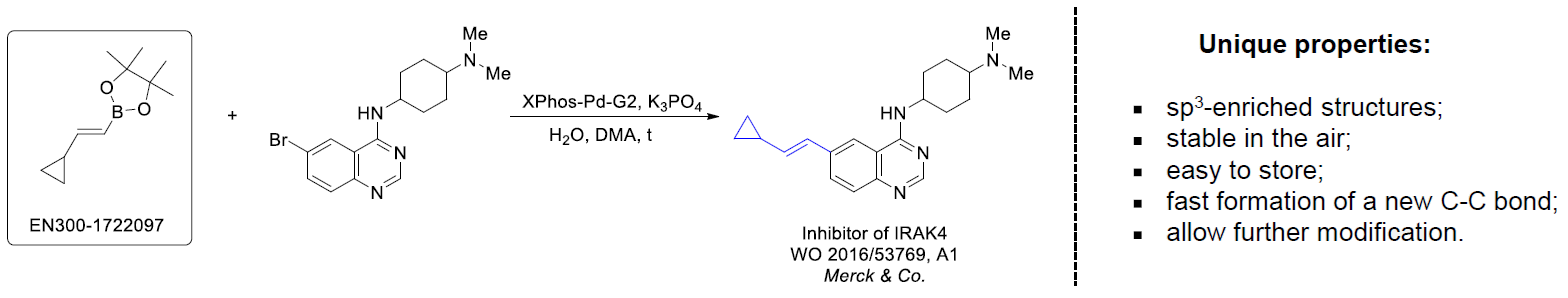
The Suzuki–Miyaura cross-coupling of boronic acid derivatives is one of the most used reactions in organic and medicinal chemist's toolbox. The rapid advancement of this method resulted in its efficient application for the late-stage modification of biologically active substrates and construction of combinatorial libraries. One of the recent trends in the field of organoboron reagents is related to the shift from aromatic compounds towards cyclic sp3-enriched structures, which comply with criteria of lead-oriented synthesis. In this context, Enamine offers a library of alkenylboronic esters for metal-mediated couplings.
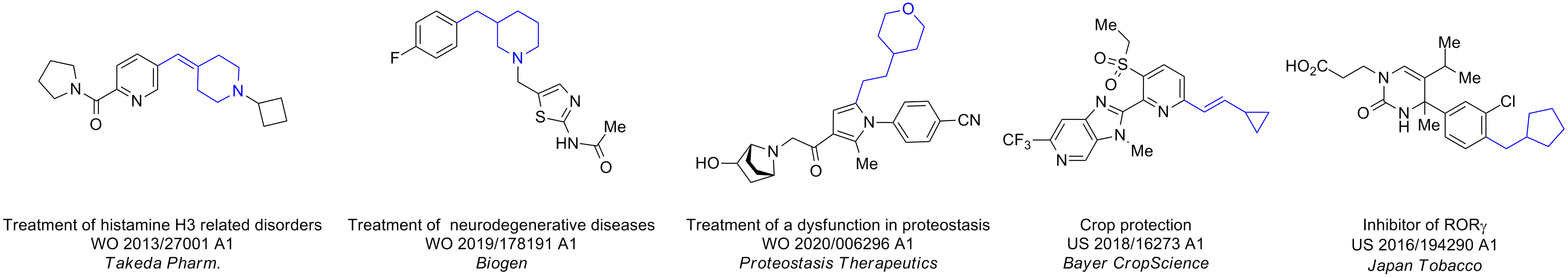
Case studies
Download SD file
Download PDF file
We offer:
more than 50 of vinyl boronates from stock on a 5-10 g scale.