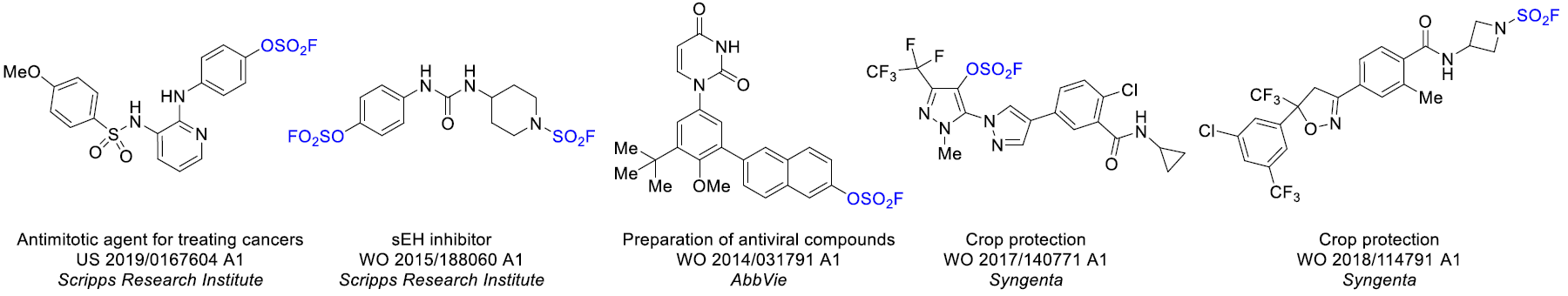
Aryl fluorosulfates and sulfamoyl fluorides are widely used in chemical biology, medicinal chemistry and agrochemistry. The former exhibit chemoselective reactivity with the side chains of tyrosine, lysine, serine and histidine in the proteins, and can be used to target non-enzymes as well as enzymes. Depending on the nature of the substituent, the -OSO2F unit can be a good leaving group or a robust connector. The fluorosulfates are quite stable toward hydrolysis under neutral or acidic conditions. The N-disubstituted sulfamoyl fluorides are stable toward hydrolysis under basic condition, inert toward a wide range of nucleophiles and dramatically more robust than analogous chlorides. In this context, Enamine offers a library of unique aryl fluorosulfates and sulfamoyl fluorides for drug design.
Properties of ROSO2F and R2NSO2F
Dual reactivity of fluorosulfates
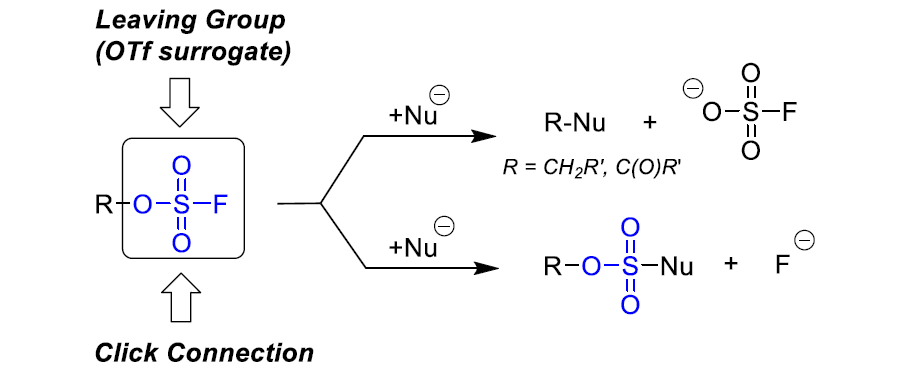
Reactivity and stability of the sulfamoyl fluorides
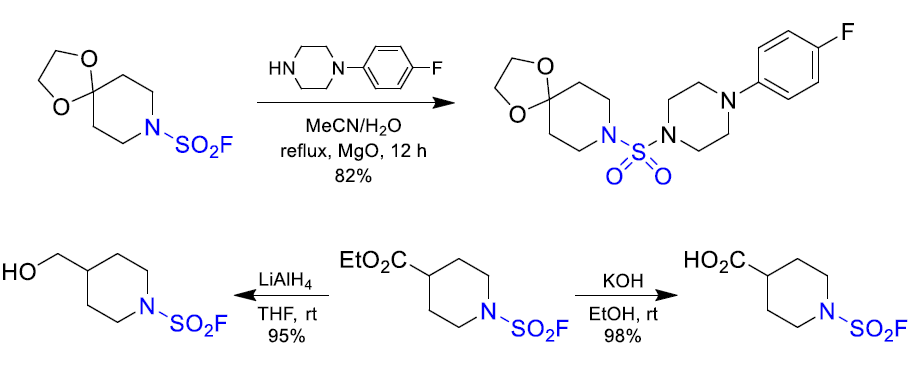
Download SD file
Download PDF file
We offer:
>100 unique fluorosulfates and sulfamoyl fluorides in gram amounts in stock.



































