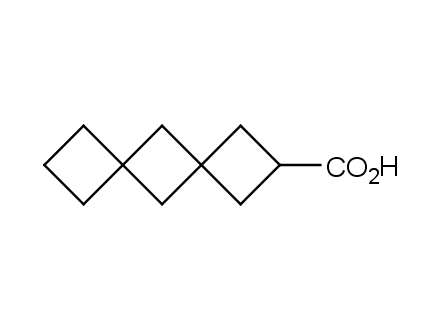
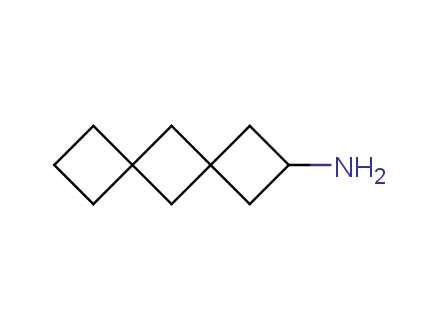
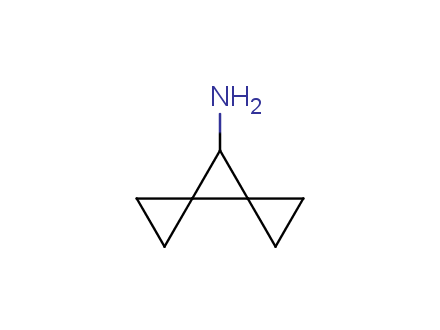
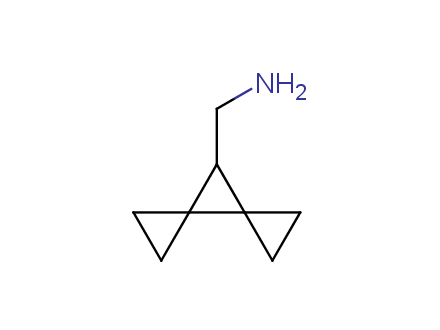
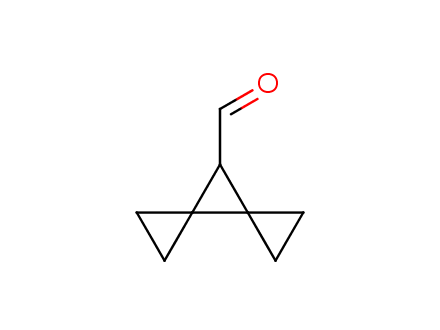
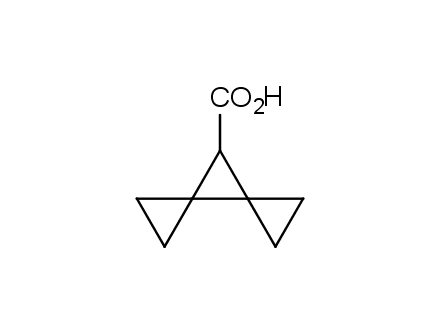
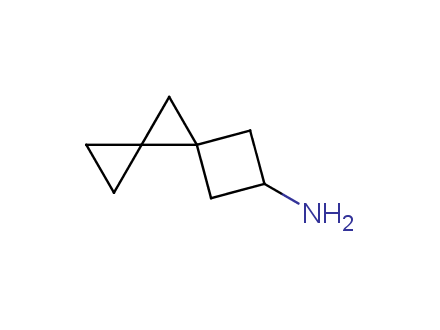
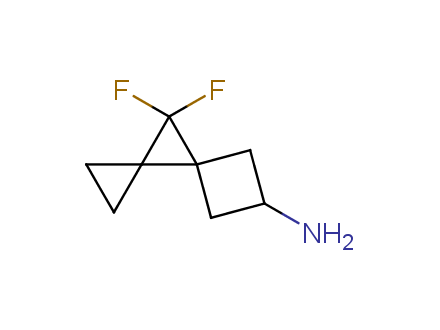
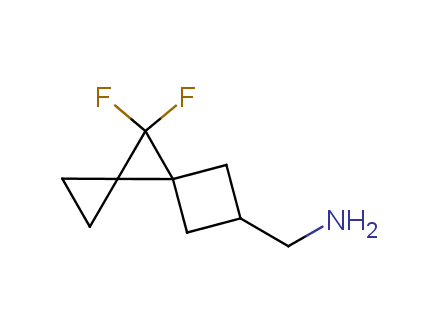
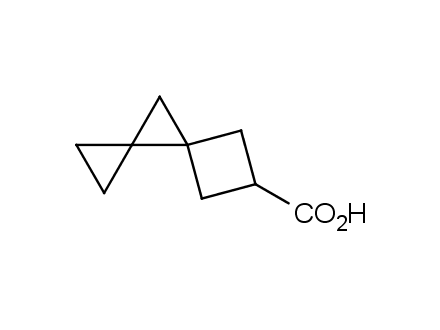
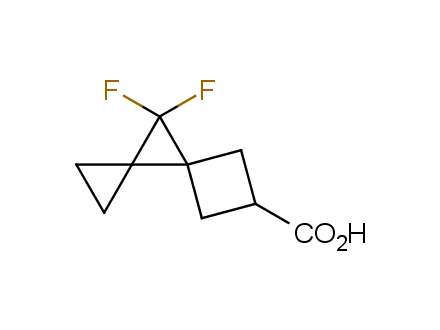
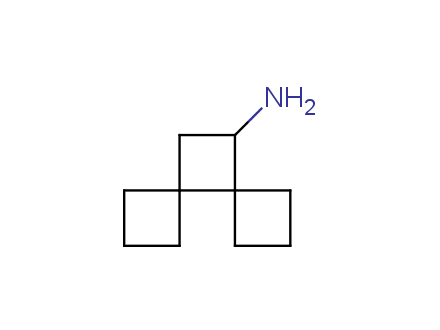
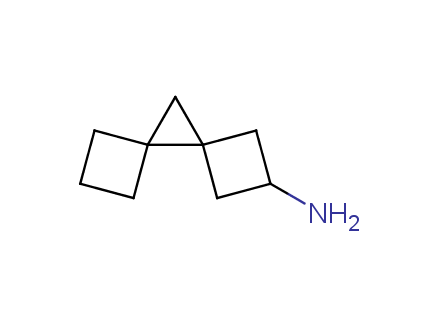
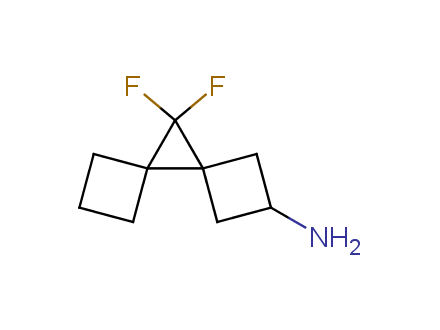
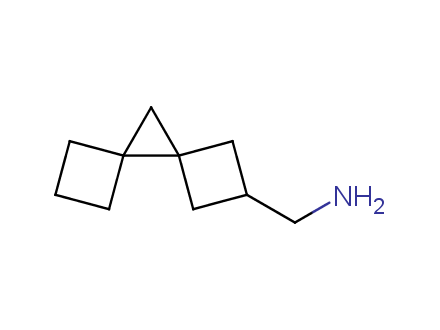
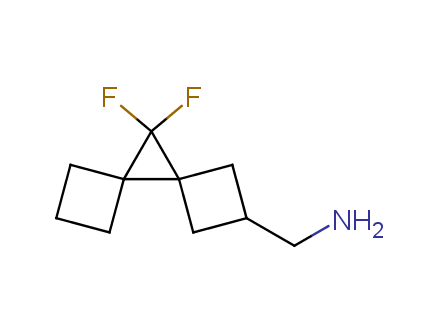
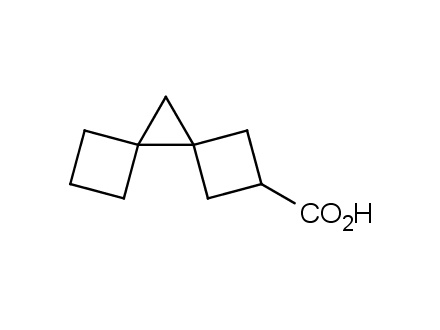
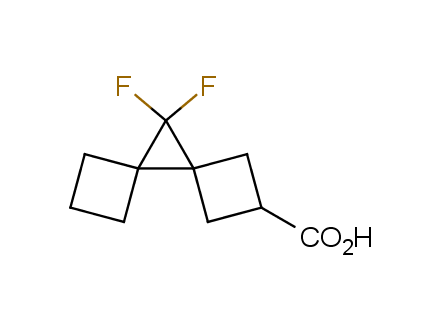
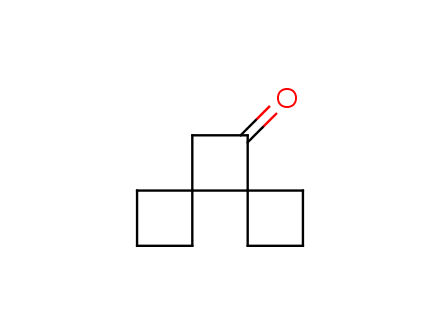
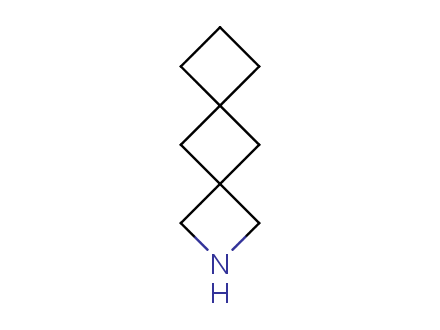
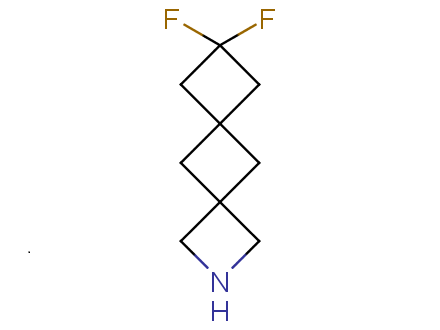
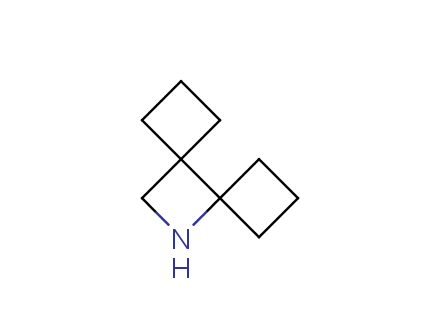
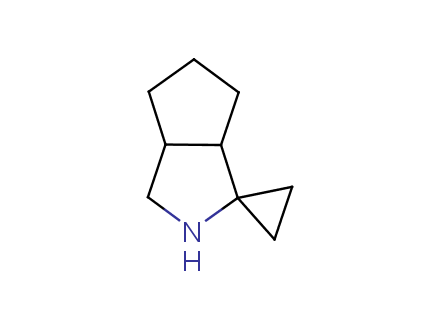
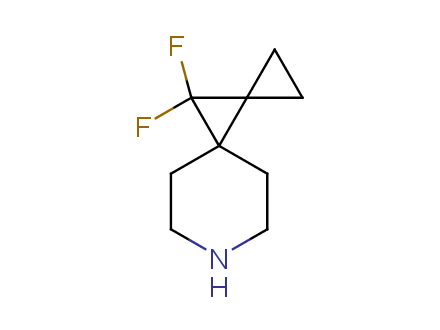
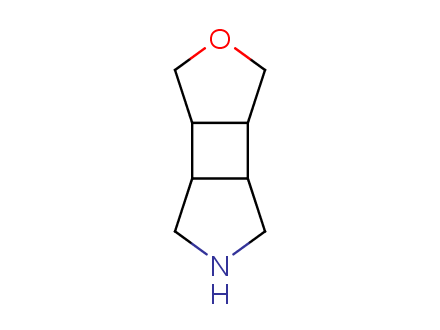
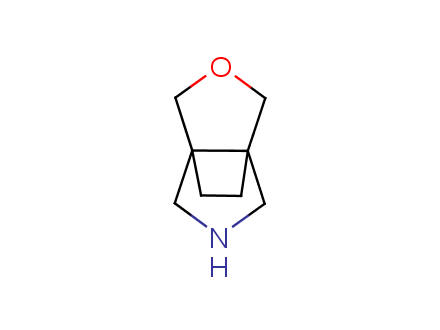
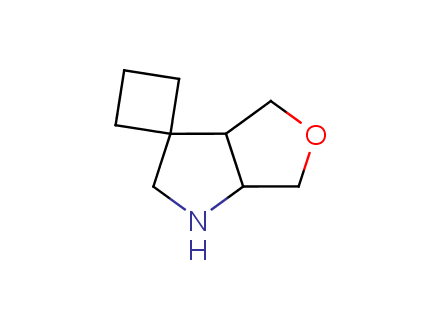
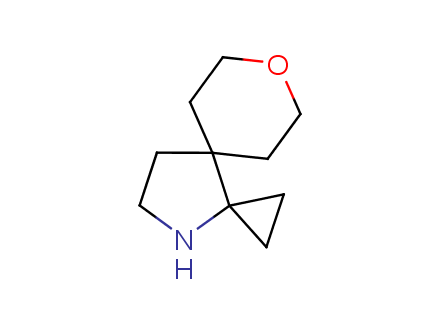
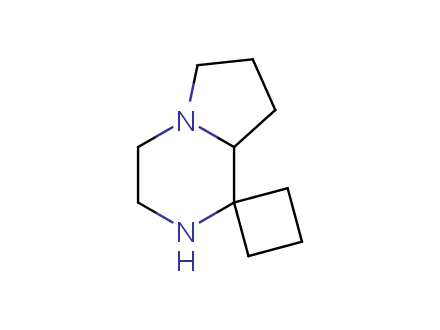
Conformational rigidification of flexible compounds by introducing a ring is a popular strategy in drug design. The resulting cyclic analogues usually have a reduced conformational entropy penalty upon binding to a protein target. A conformational restriction can also be imposed by introduction of a spirocyclic ring. Spirocyclic systems are 3D-shaped, in strict contrast to flatten benzene compounds. It is especially true for polycyclic compounds. In this context, Enamine offers a library of innovative three-cyclic scaffolds for drug design.
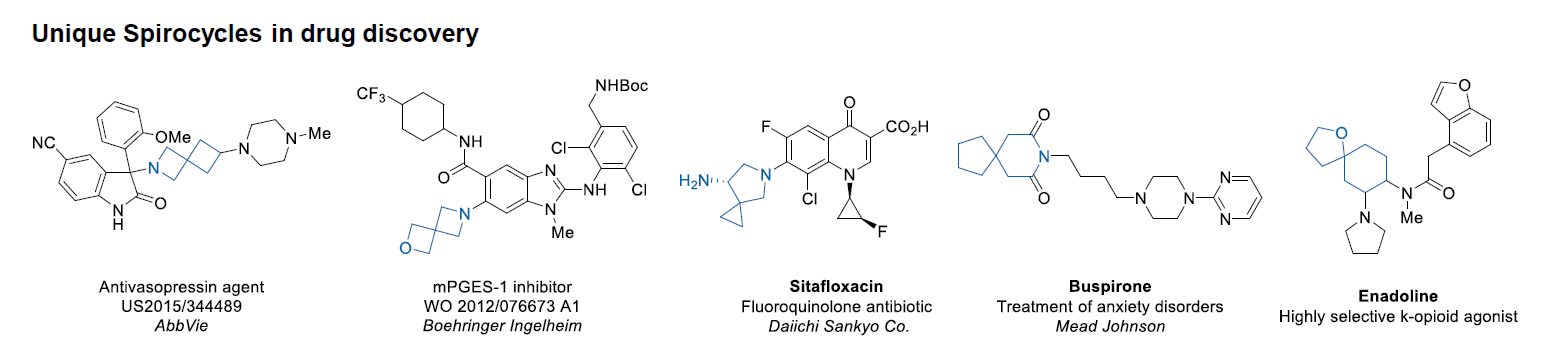
Case studies
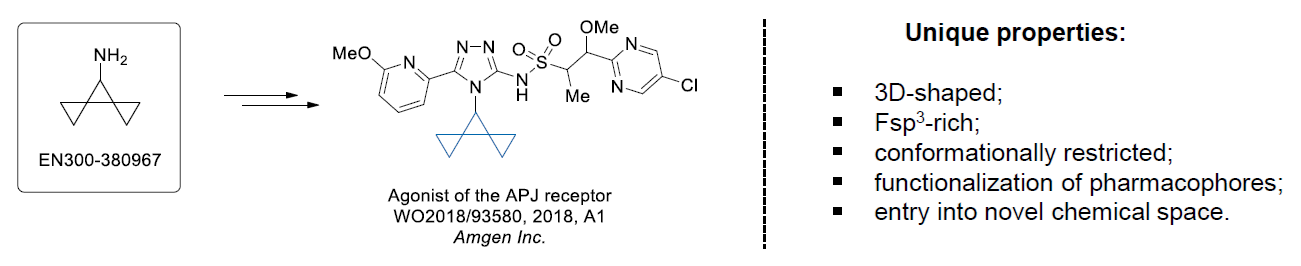
Download SD file
Download PDF file
We offer:
More than 50 of three-cyclic building blocks from stock on a 5-10 g scale