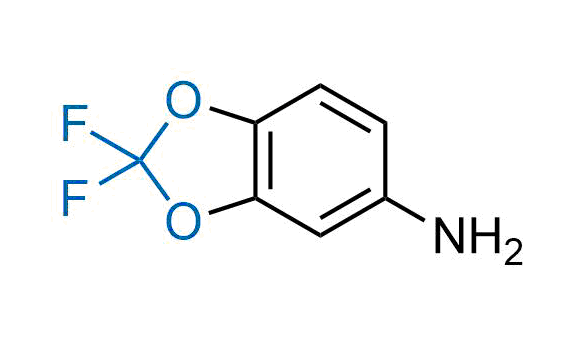
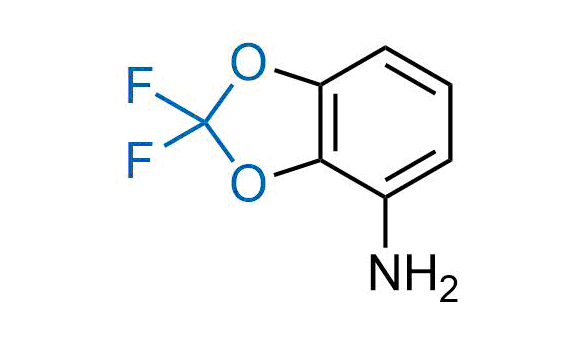
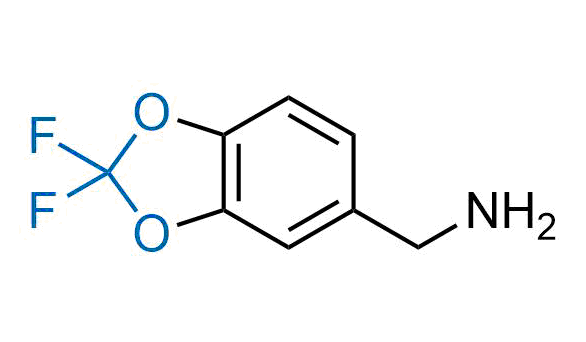
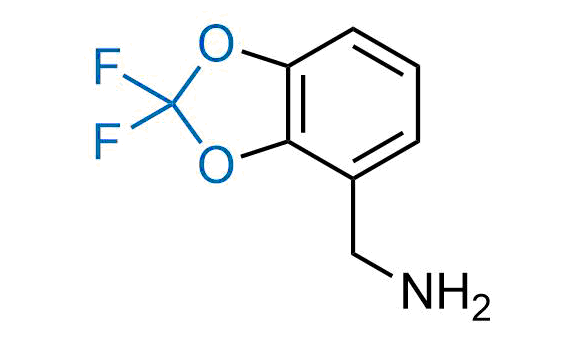
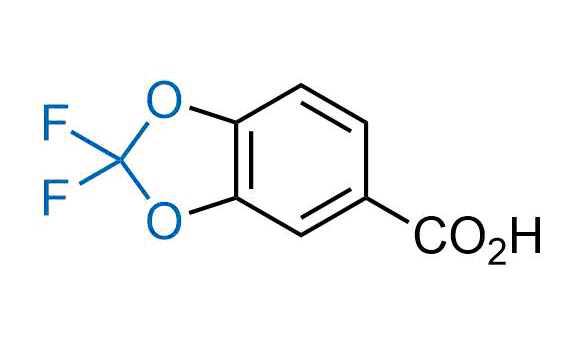
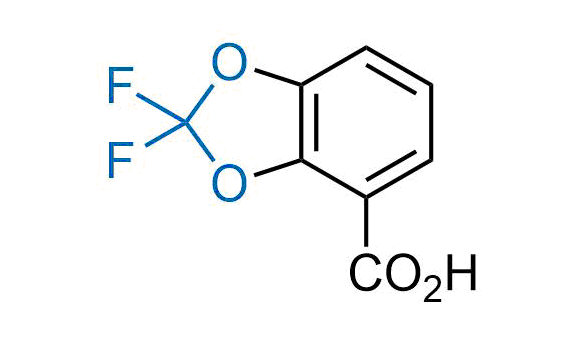
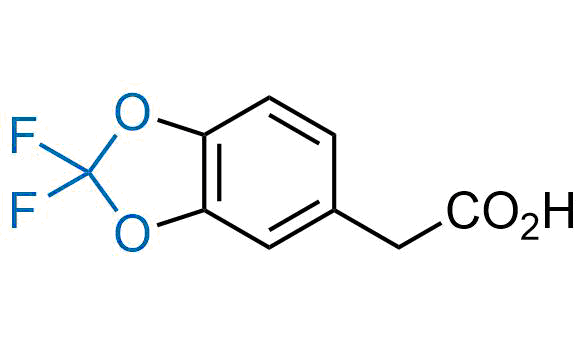
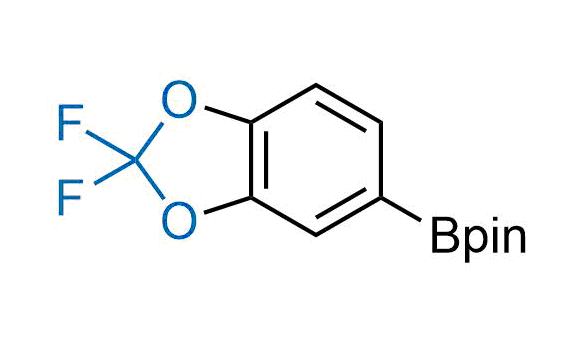
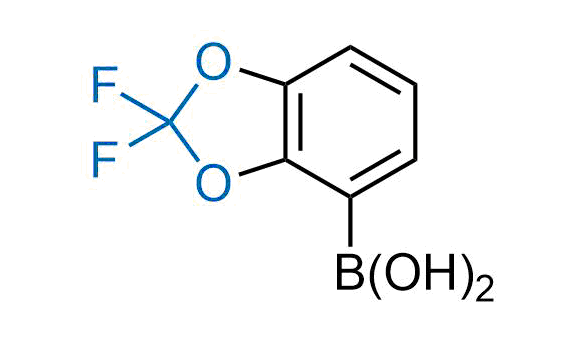
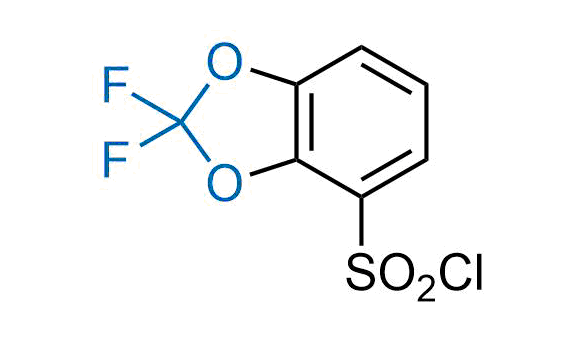
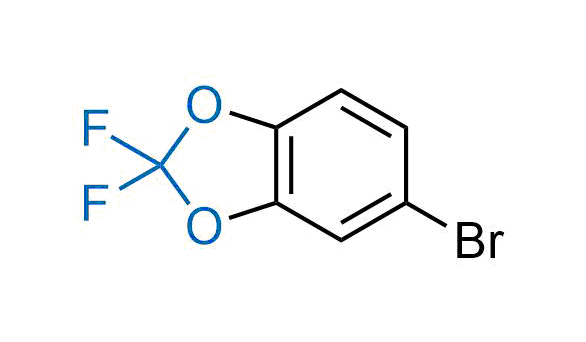
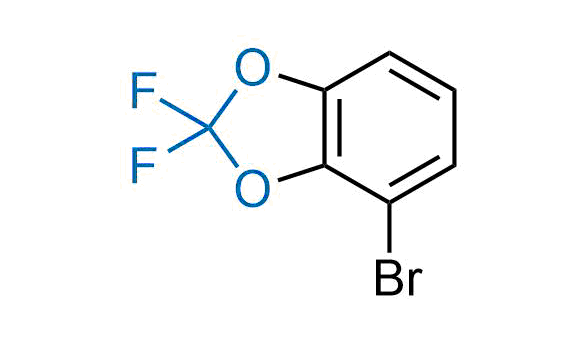
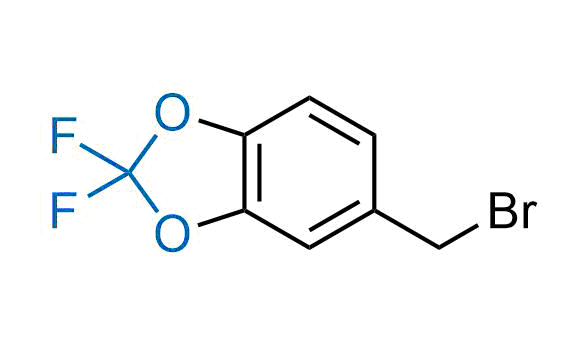
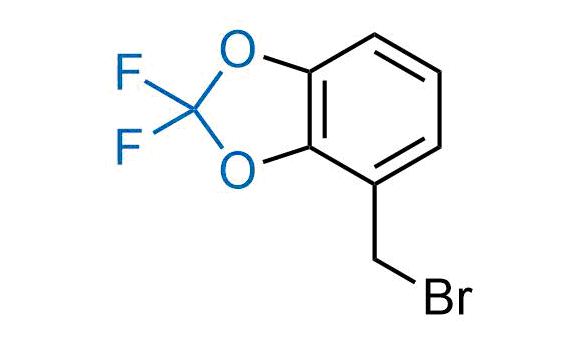
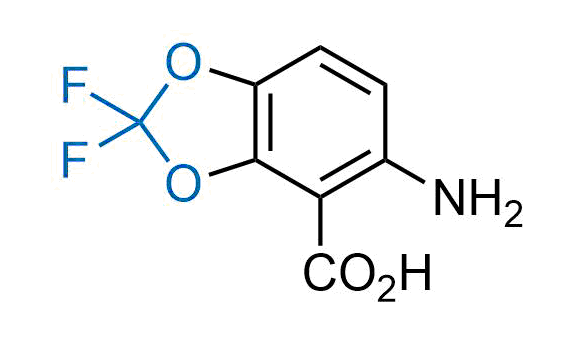
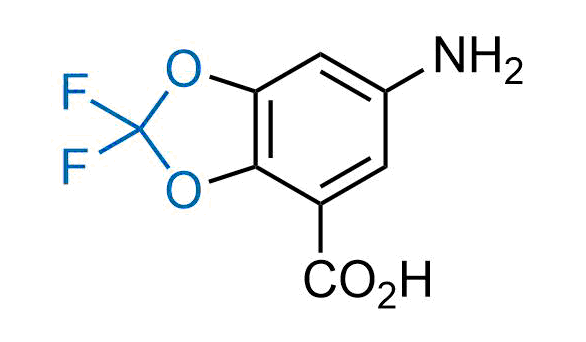
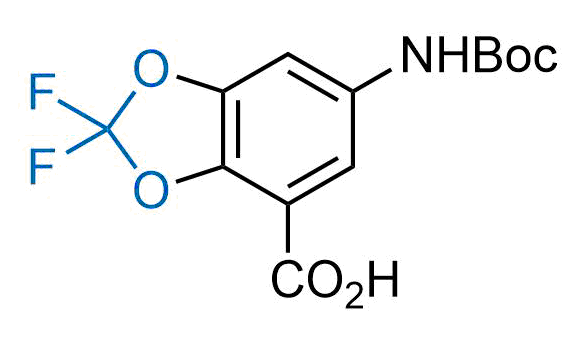
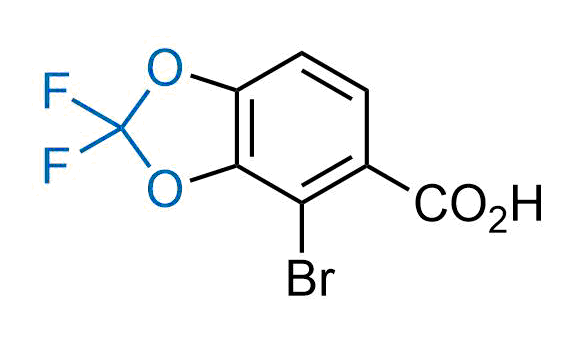
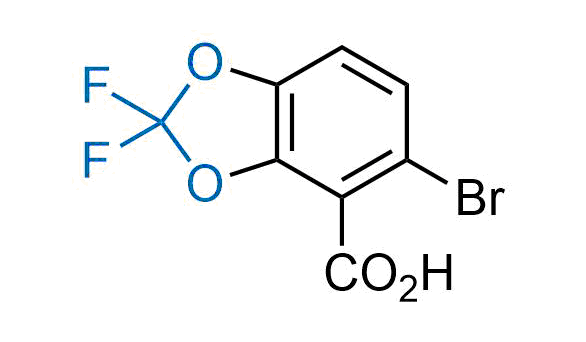
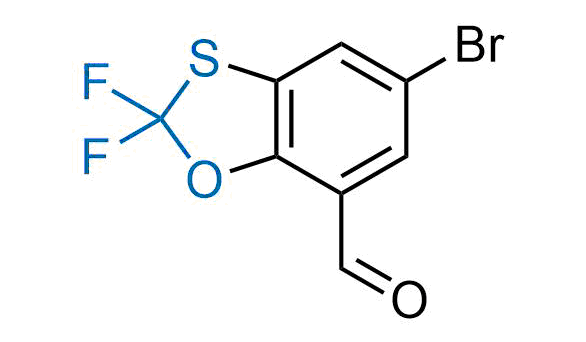
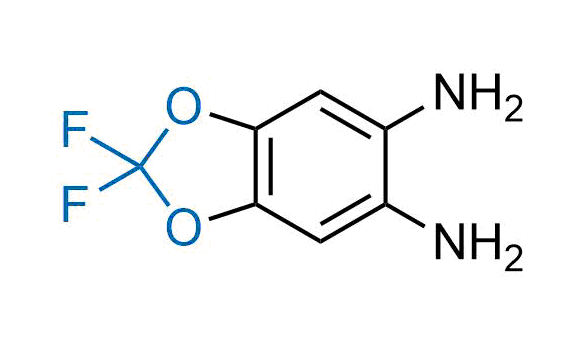
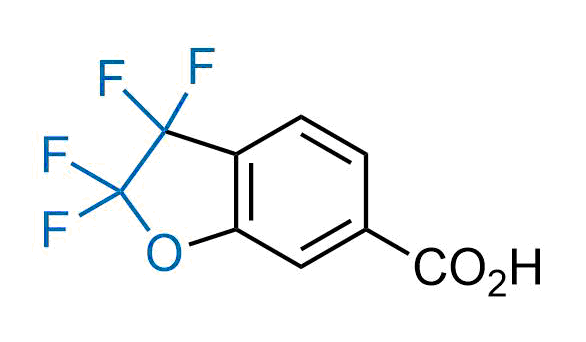
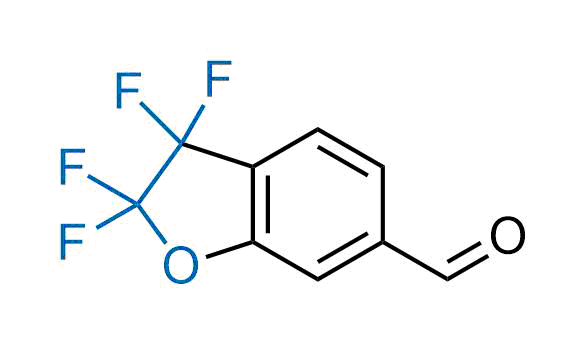
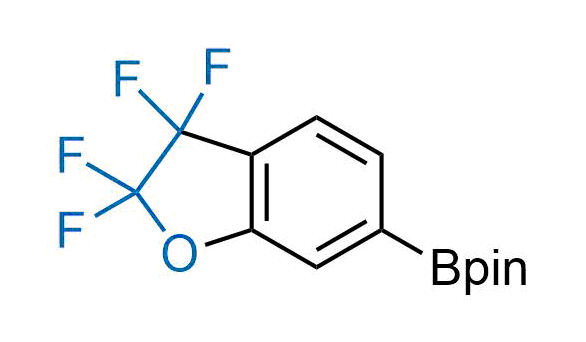
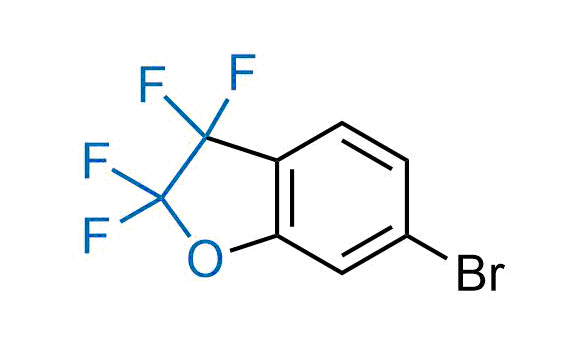
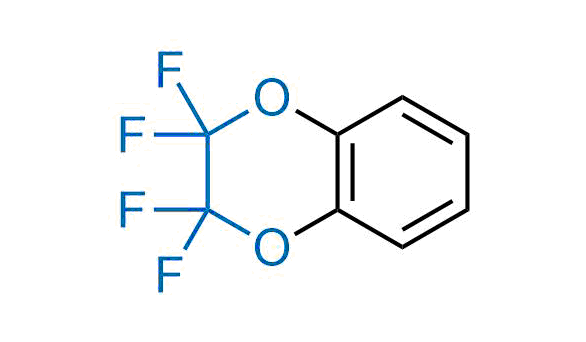
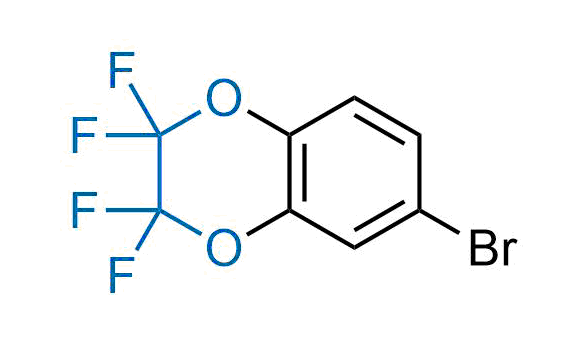
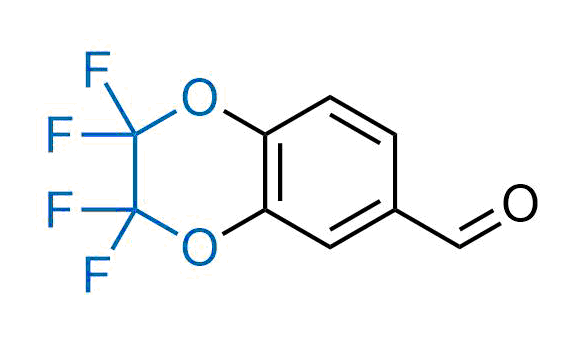
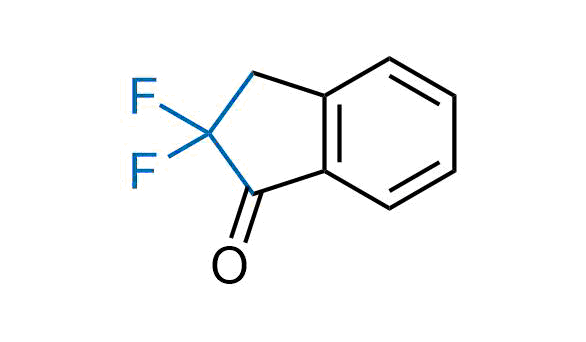
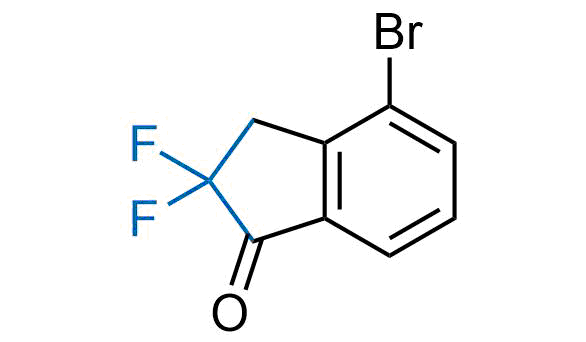
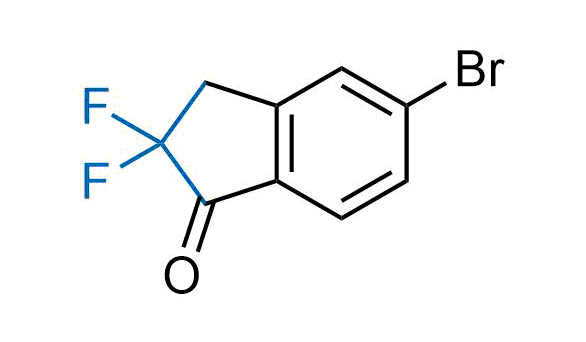
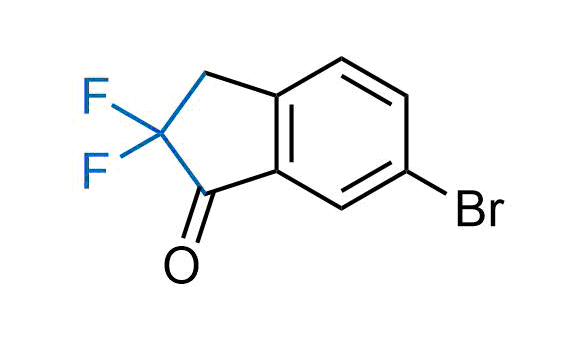
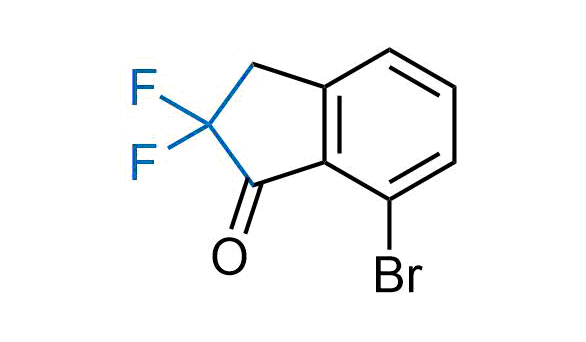
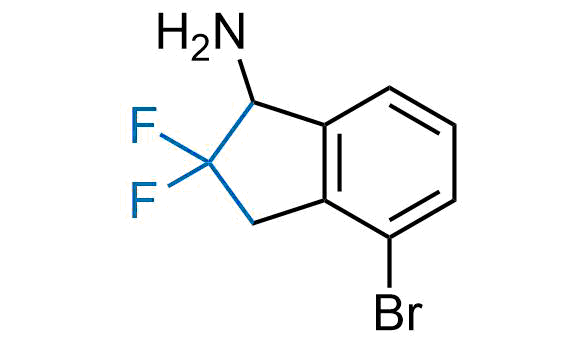
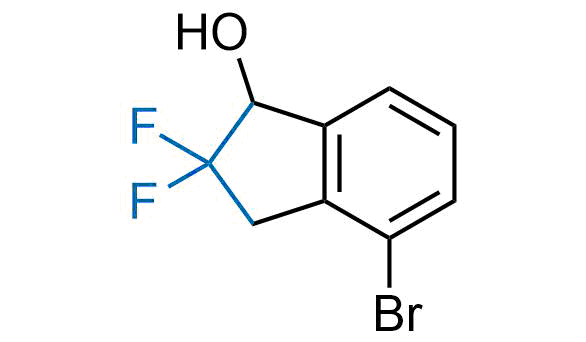
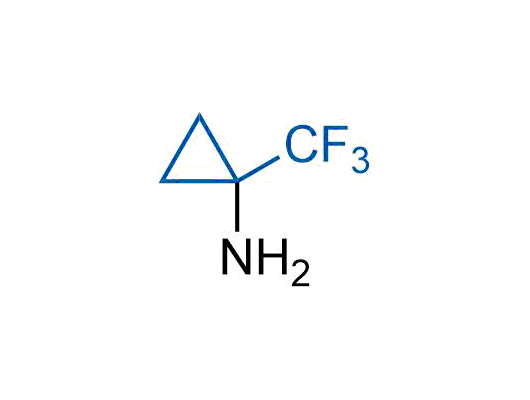
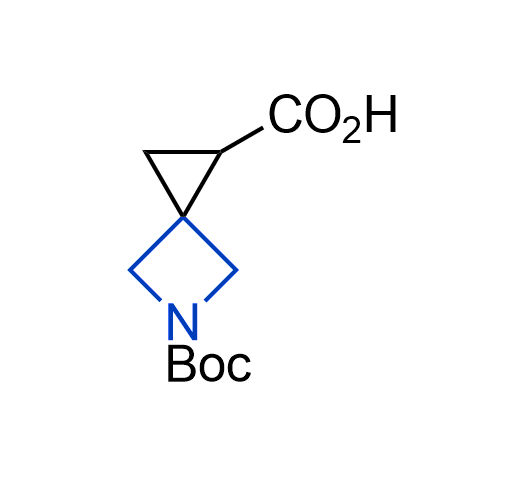
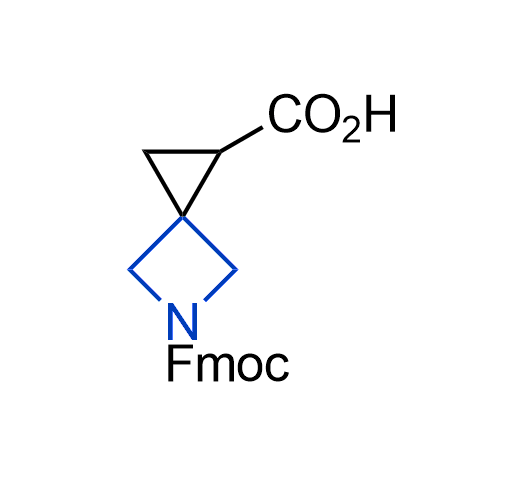
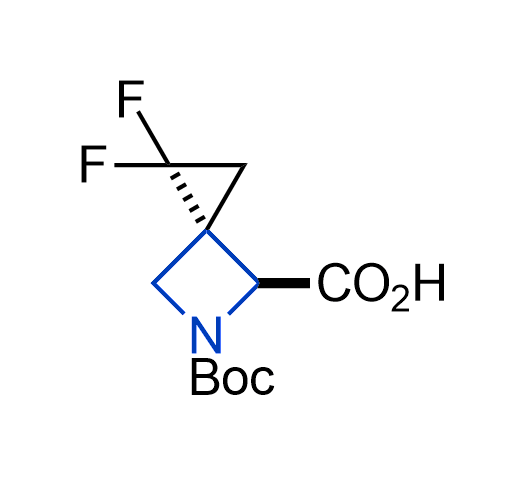
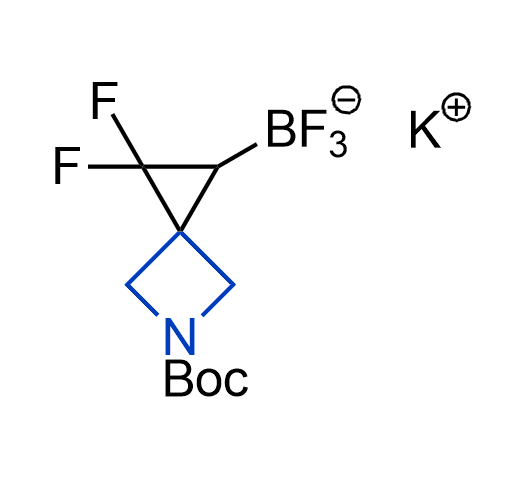
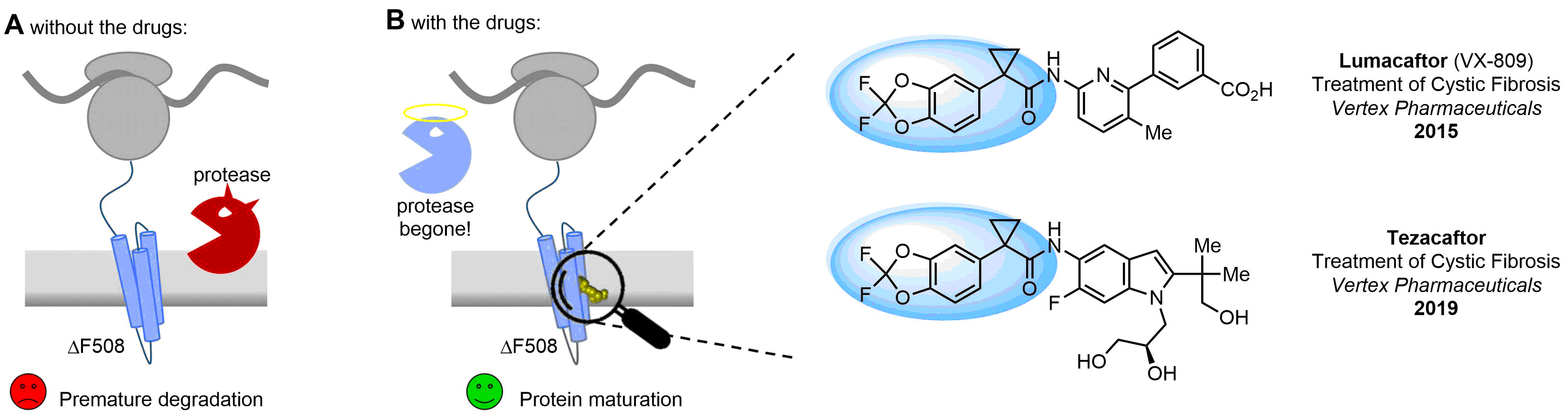
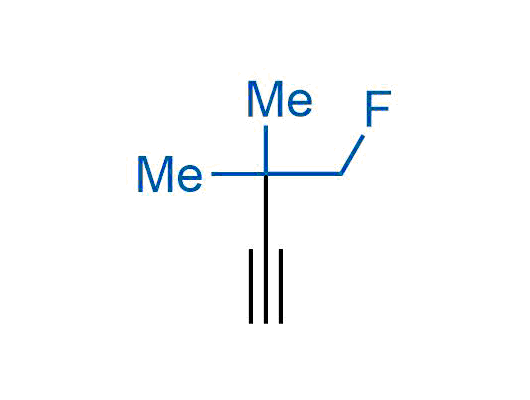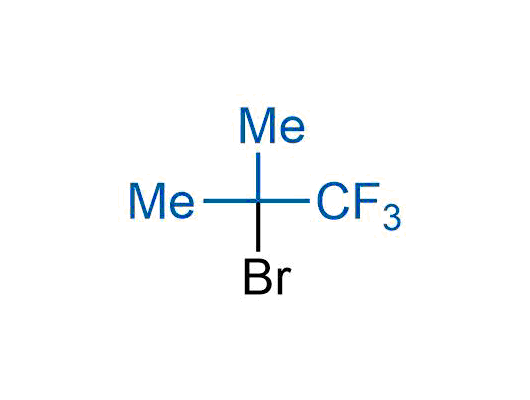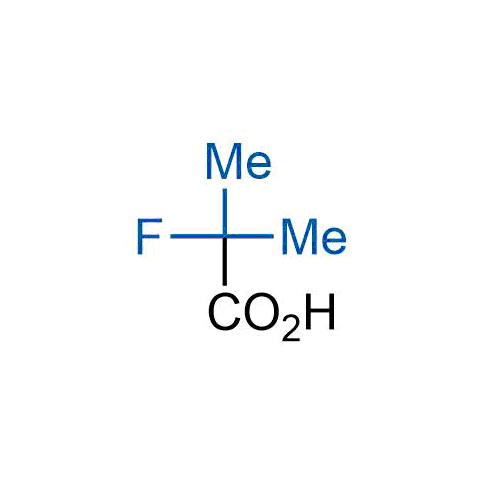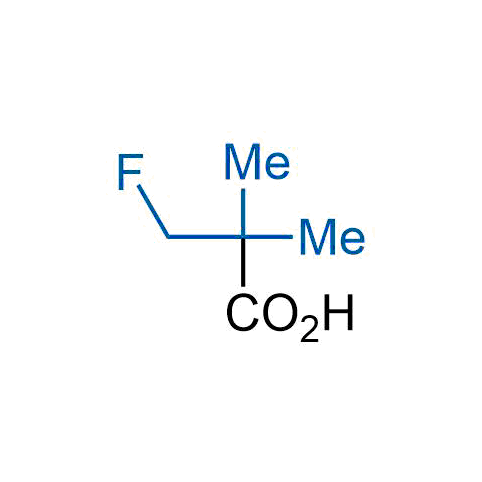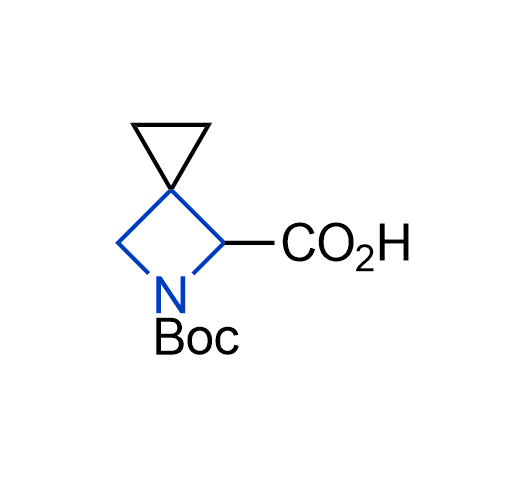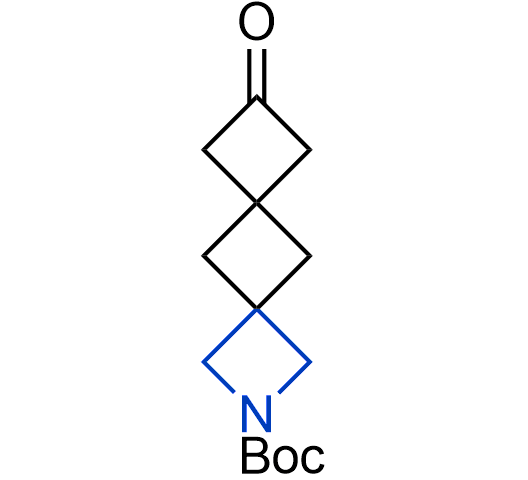Drugs with the benzodioxole moiety showed excellent bioavailability and low cytotoxicity. The benzodioxole structure inspired the creation of fluorinated analogues to improve drug-target interactions and metabolic stability. Lumacaftor and Tezacaftor represent an innovative type of drugs: the small molecule chaperones. The difluoro-1,3-benzodioxol-5-yl-cyclopropane carboxamide group, shared between both drugs, binds to the nascent chain of the mutant protein during its biosynthesis. In this way, the protein corrects the folding defects and escapes premature degradation that could cause disease. Enamine offers a variety of advanced building blocks that share the fluorinated benzodioxole core structure.
Concept
Download SD file
Download PDF file
We offer
More than 100 fluorinated benzodioxoles and analogues from stock on a 5-10 g scale.
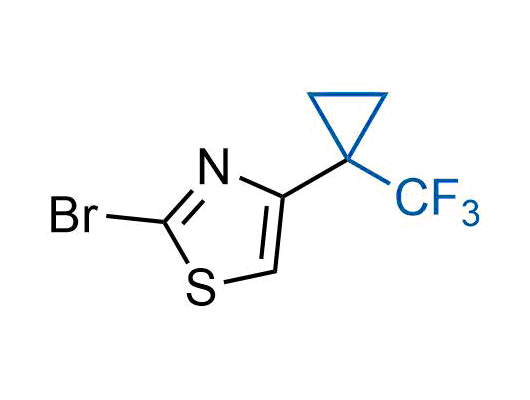
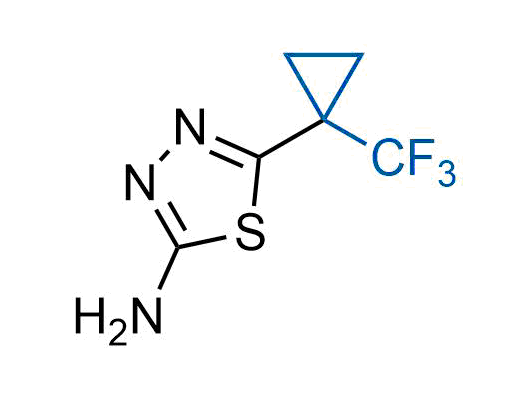
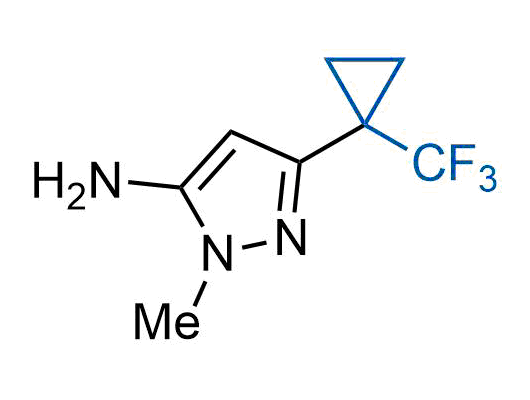
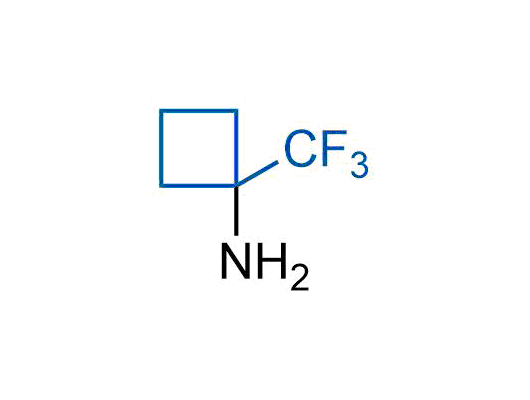
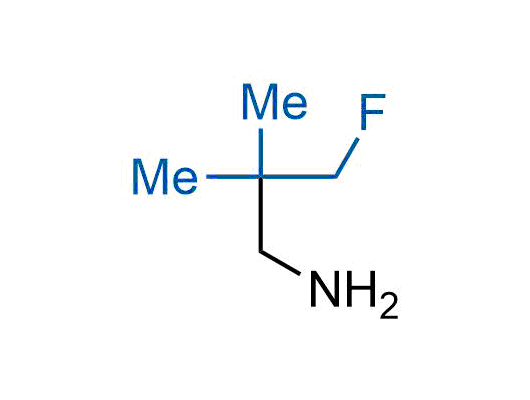
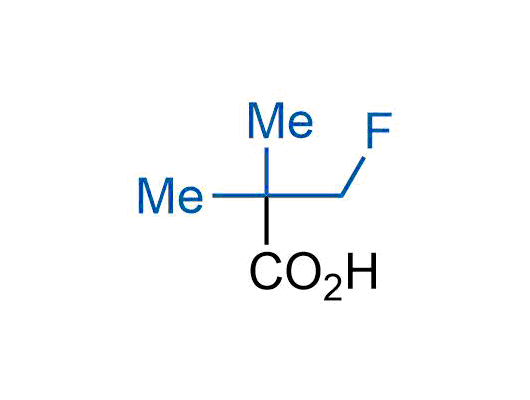
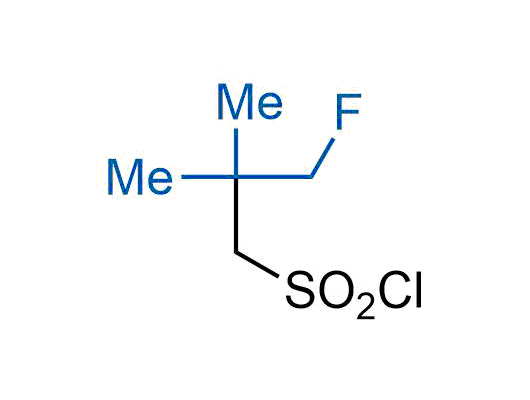
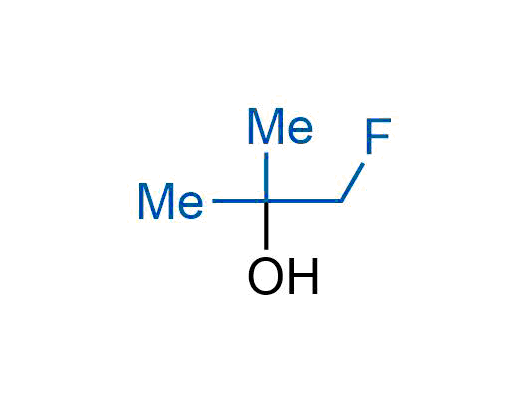
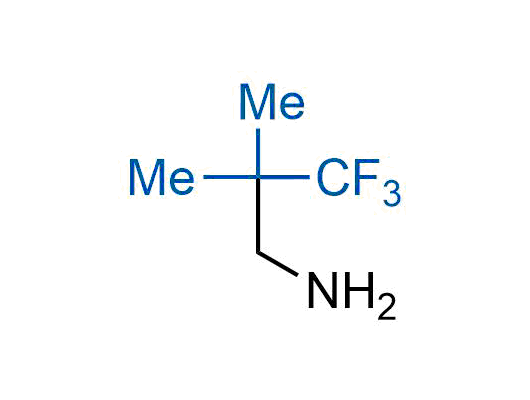
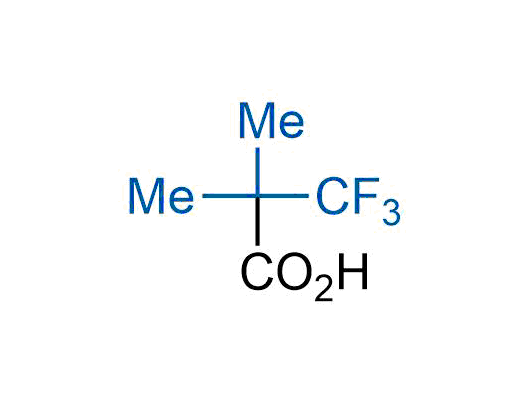
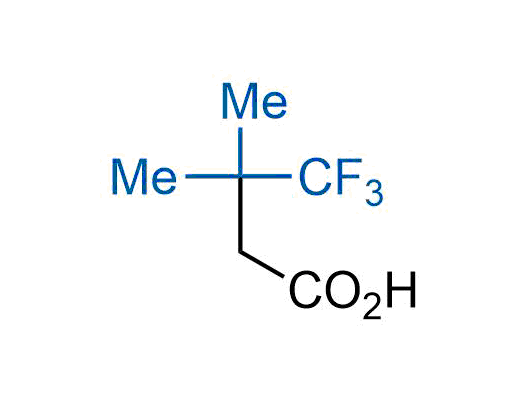
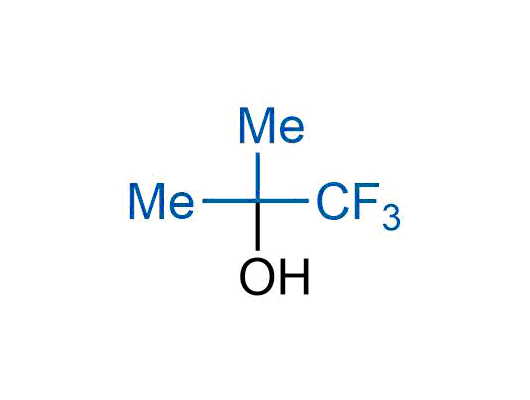
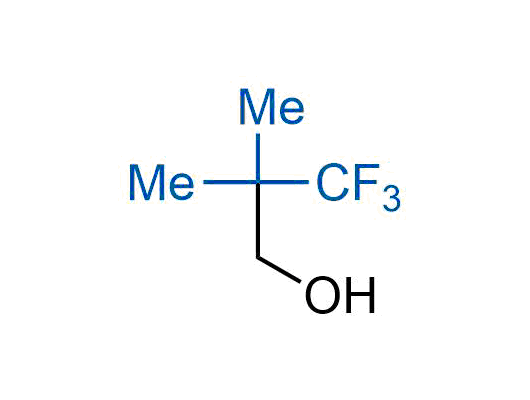
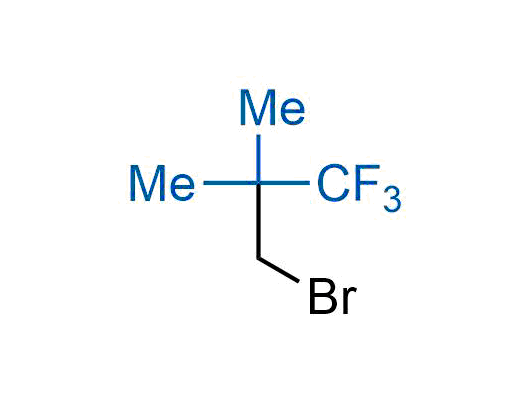
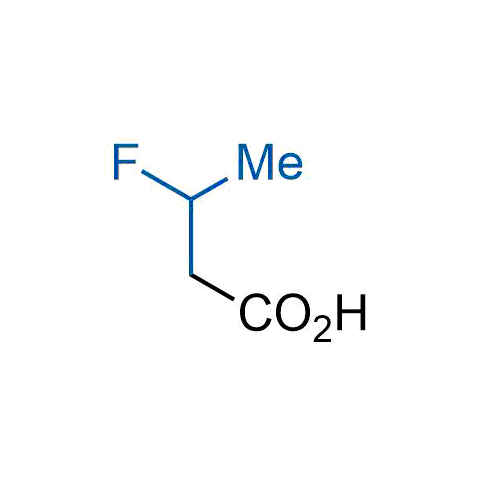
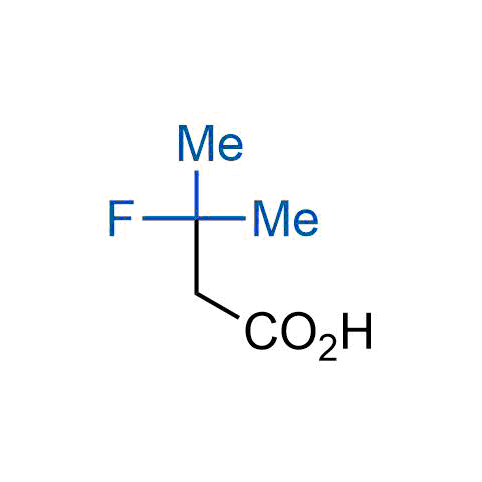
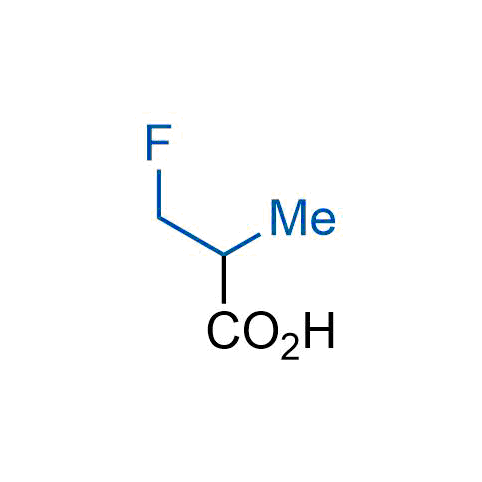
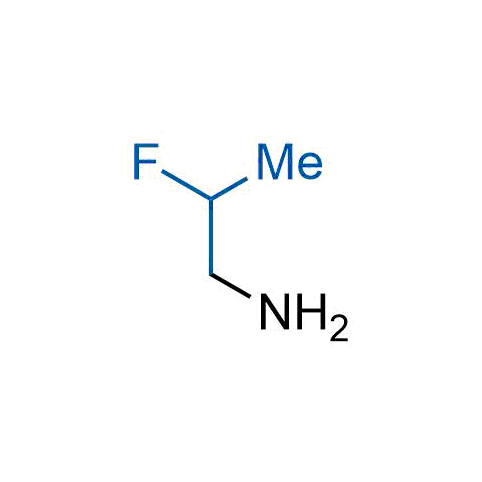
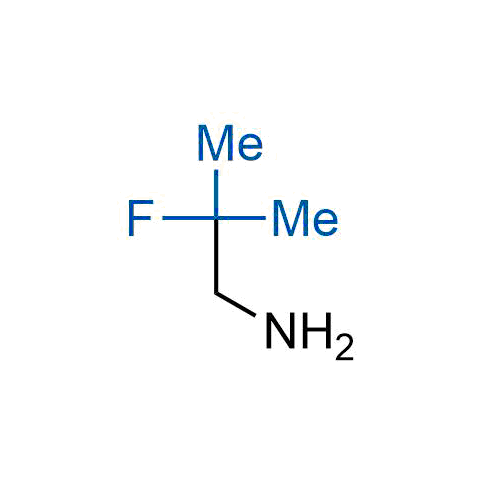
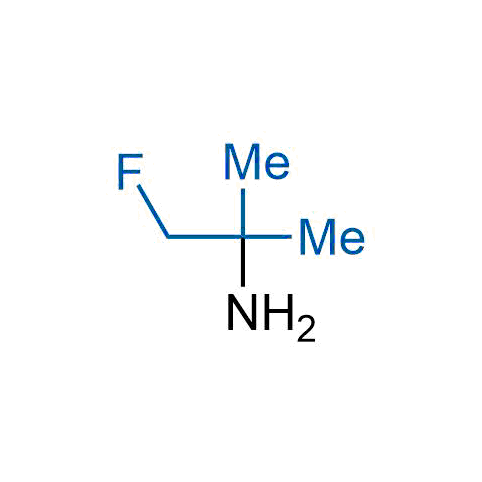
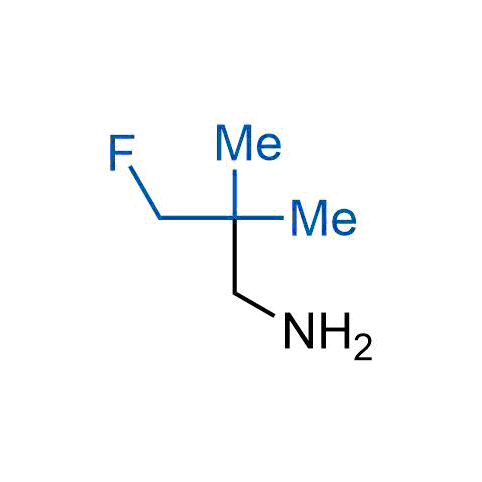
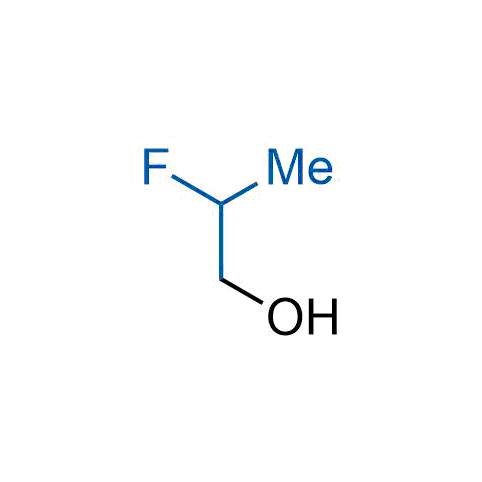
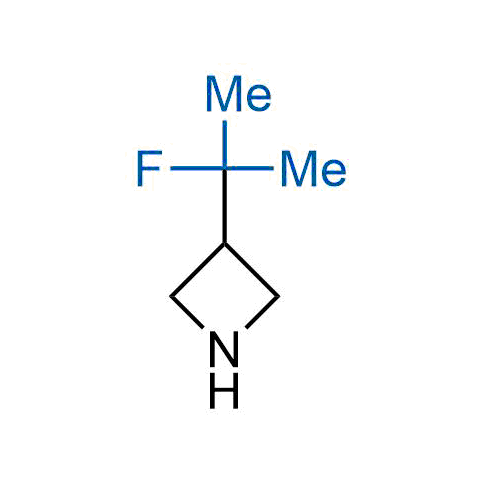
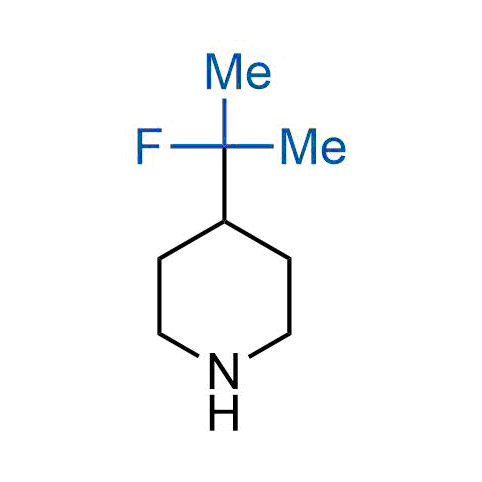
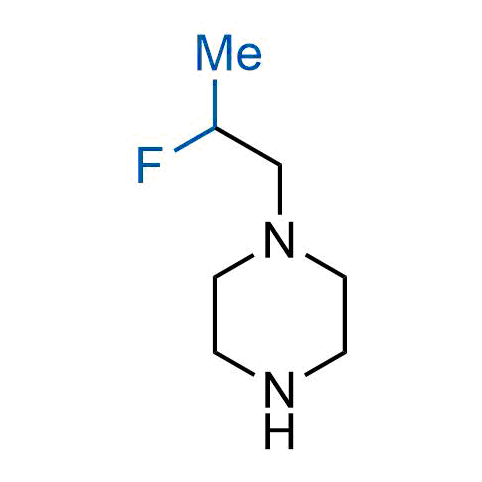
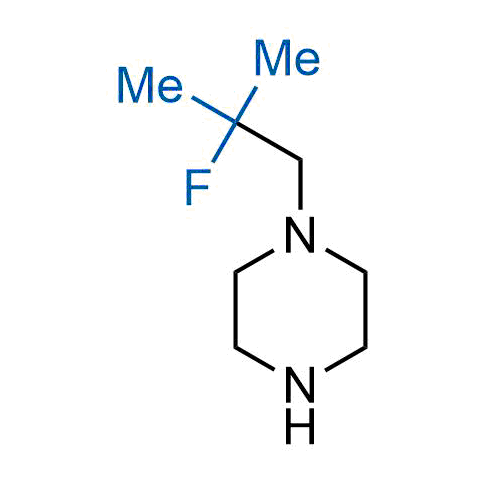
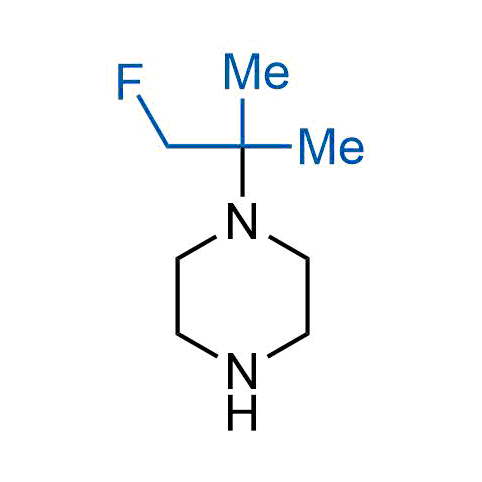
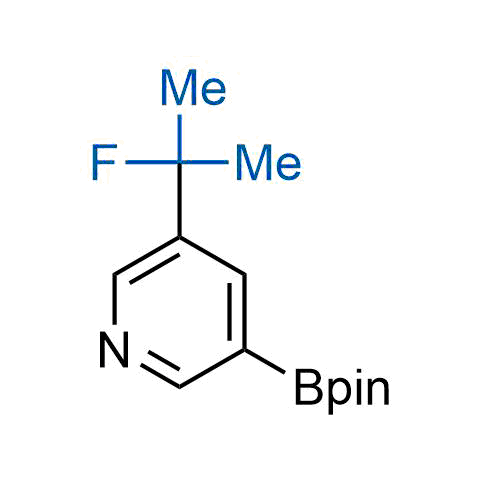
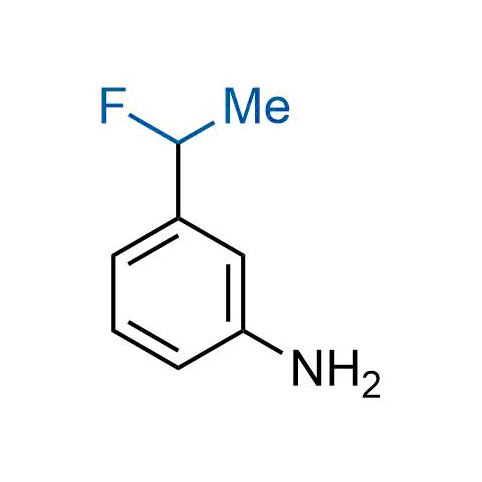
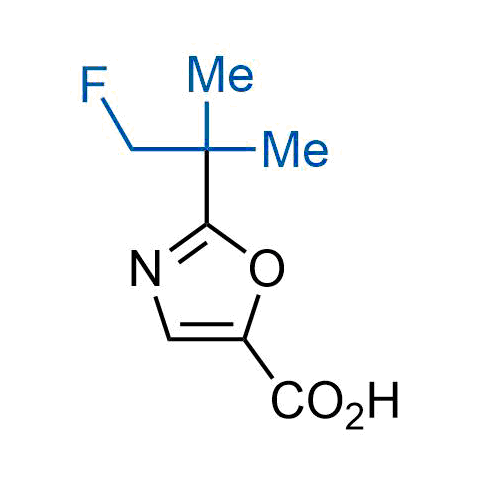
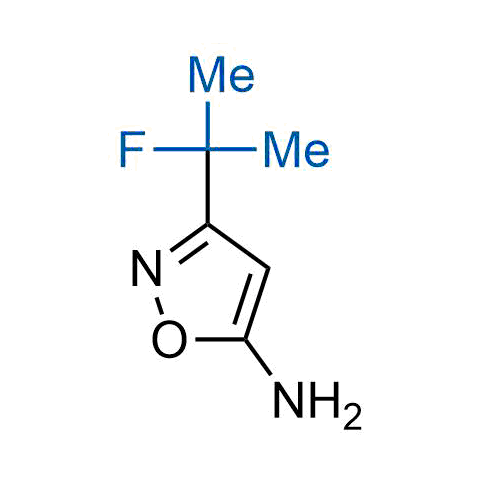
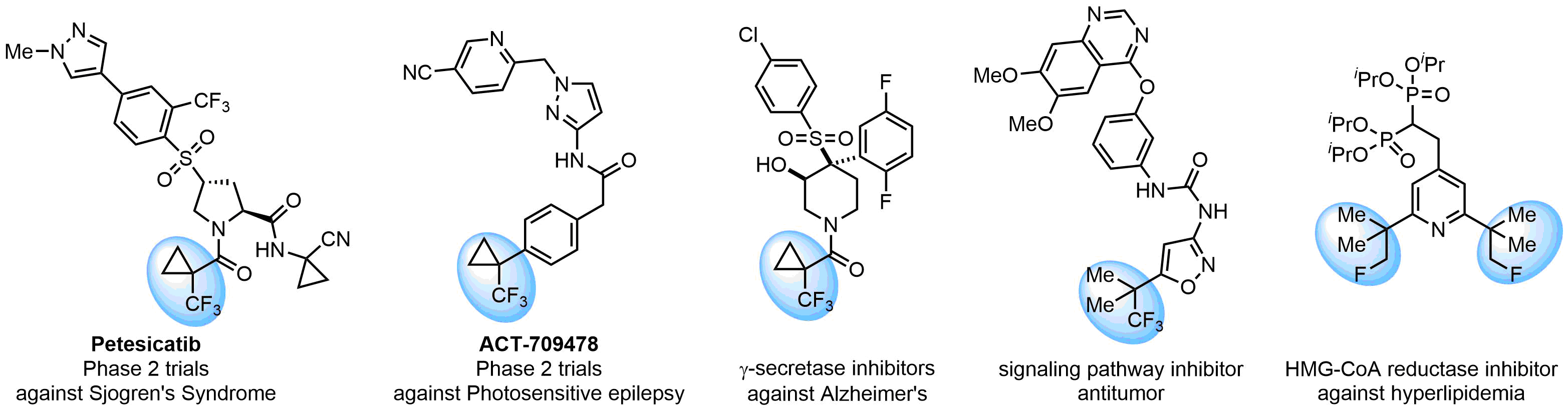
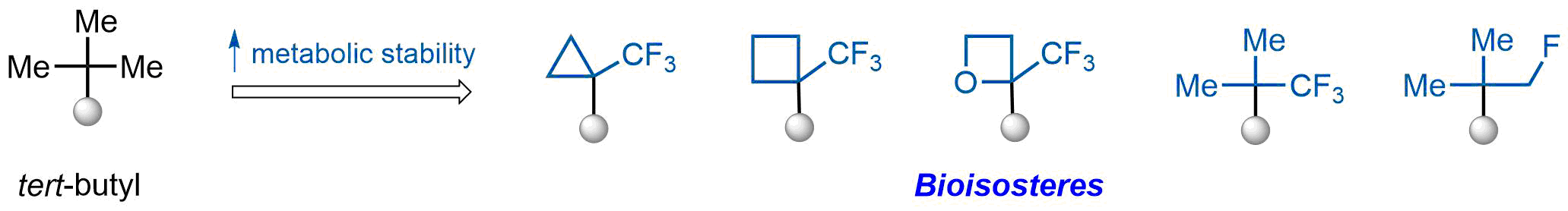
The tert-butyl group (-C(CH3)3) is a common motif in drug design due to its steric and electronic properties. Its bulky size and non-polar nature can be mimicked by other groups. Examples of bioisosteres of the tert-butyl group include cyclopropyl, cyclobutyl, isopropyl, and fluorine. These compounds can be used to modify the lipophilicity, solubility, and pharmacokinetic properties of a drug molecule to improve its efficacy and safety.
Concept
Download SD file
Download PDF file
We offer
More than 100 bioisosteres of tert-butyl group from stock on a 5-10 g scale.
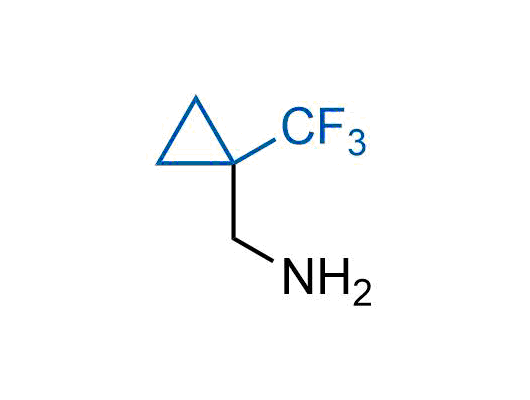
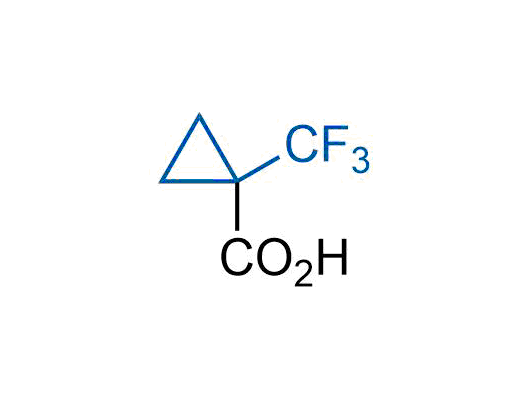
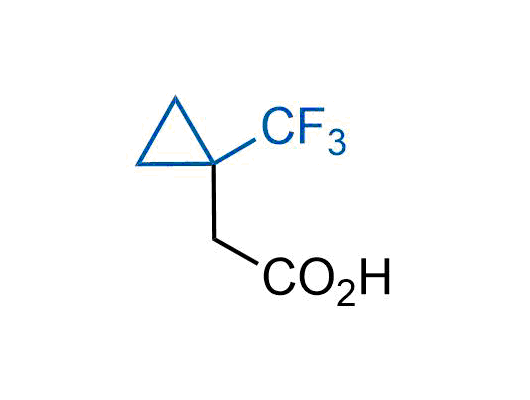
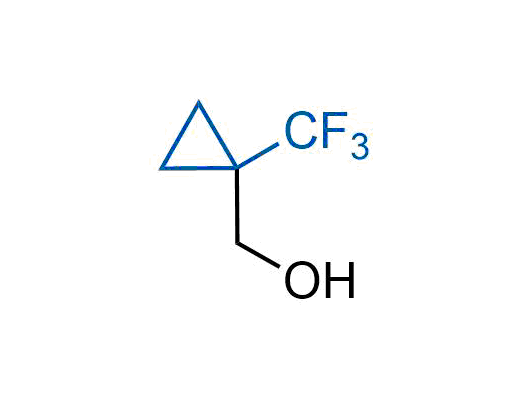
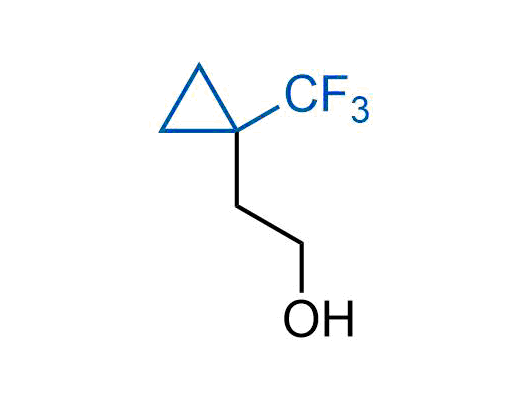
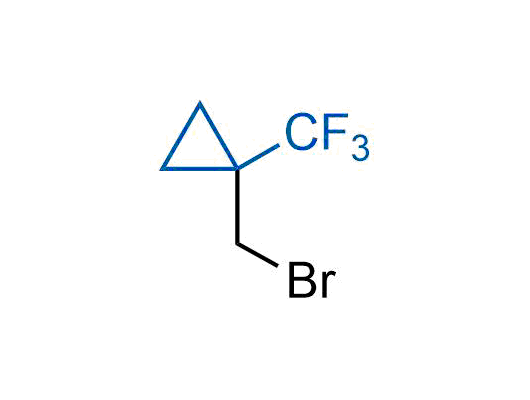
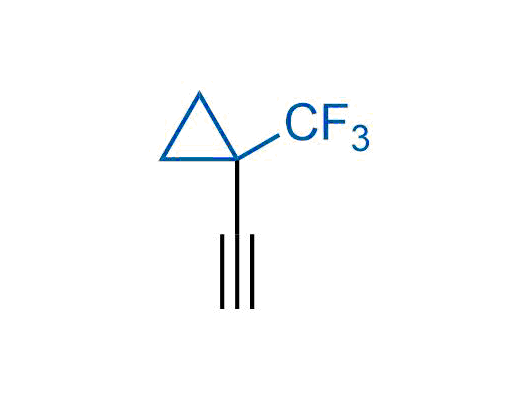
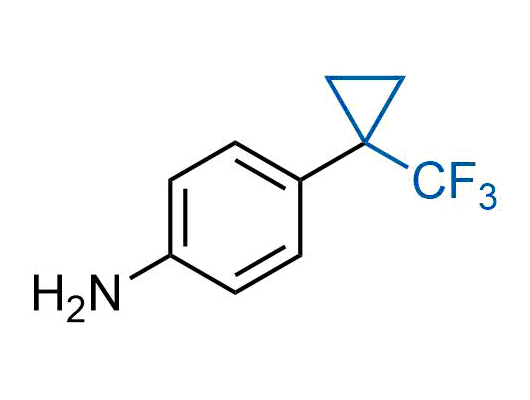
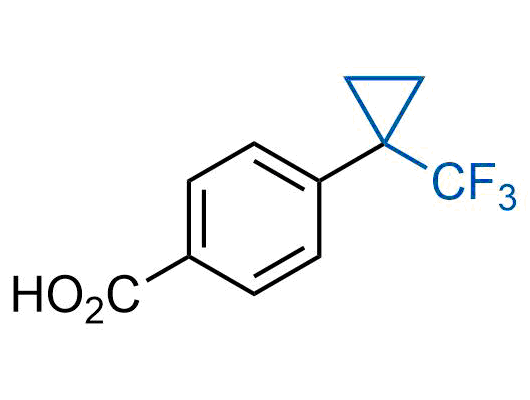
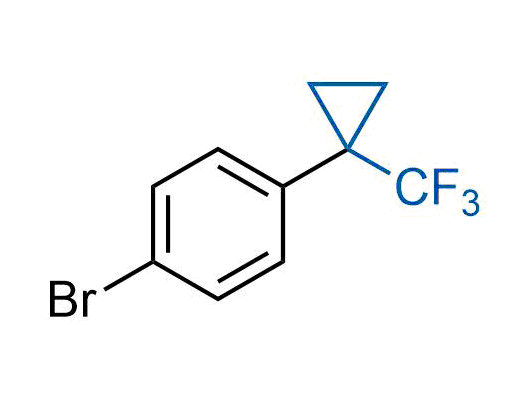
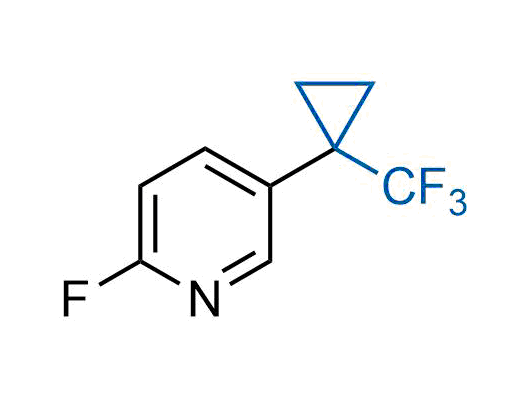
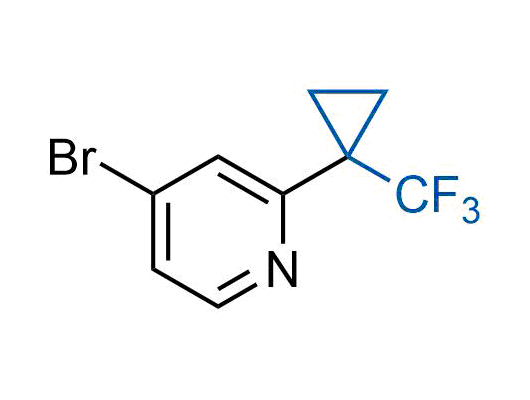
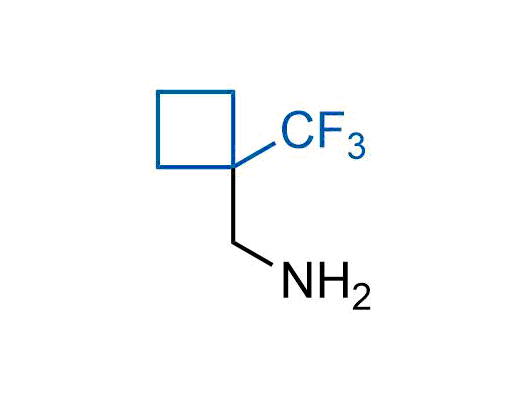
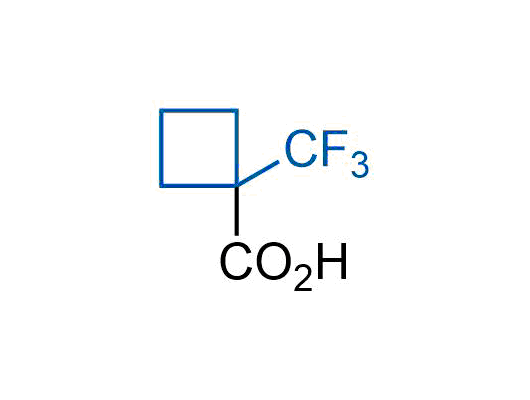
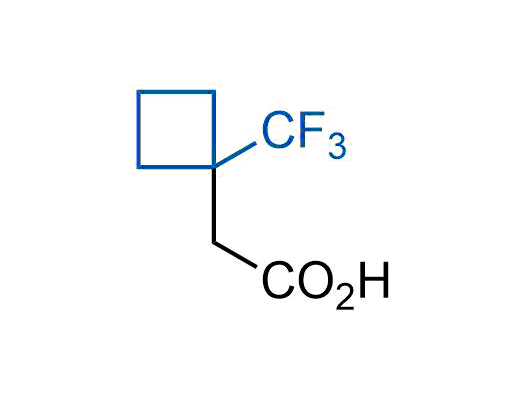
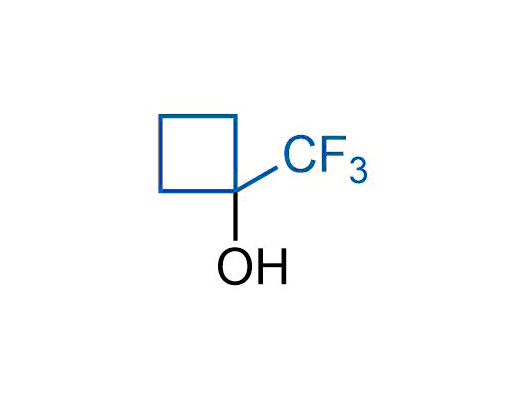
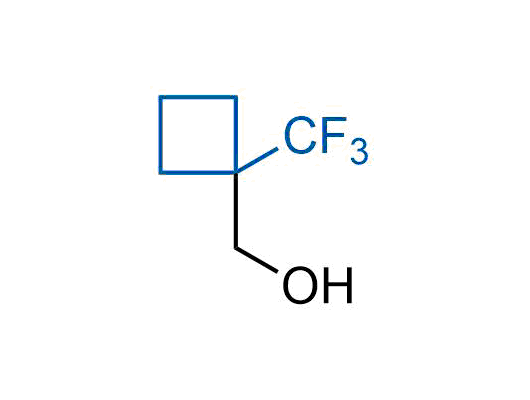
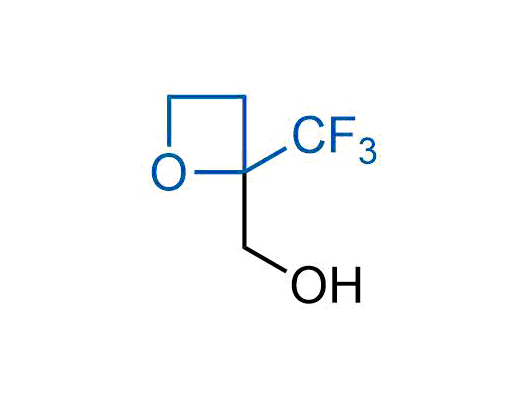
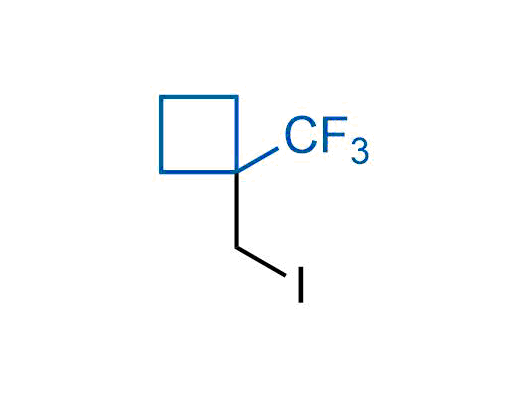
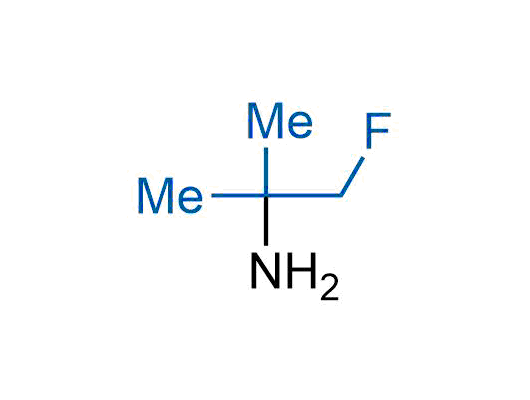
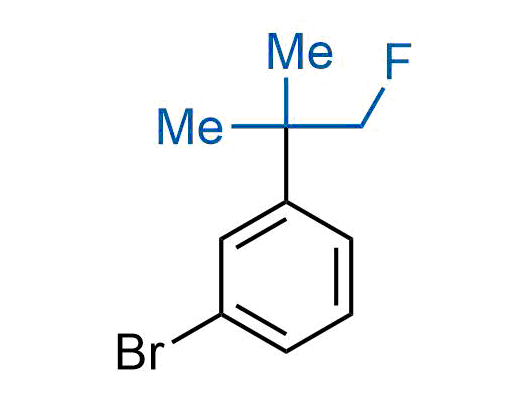
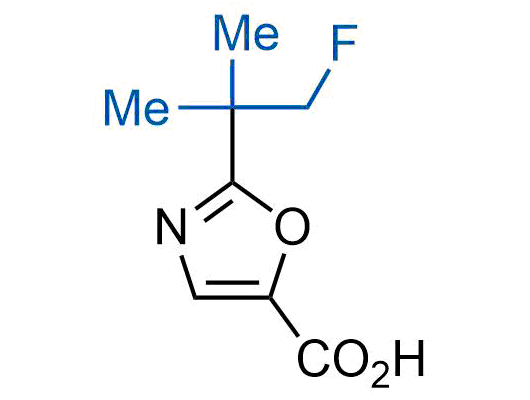
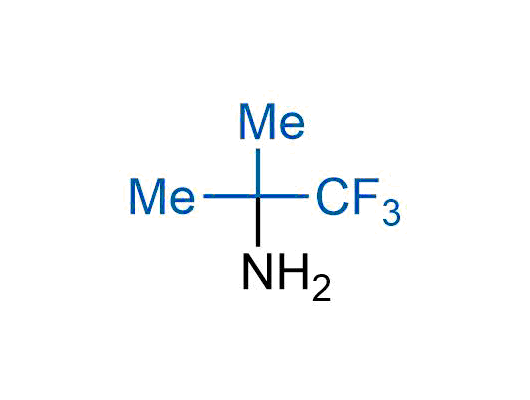
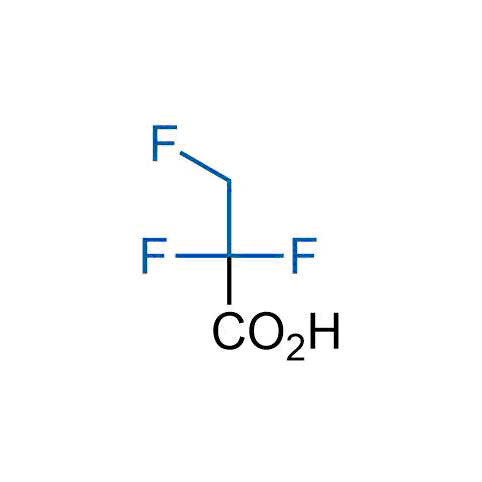
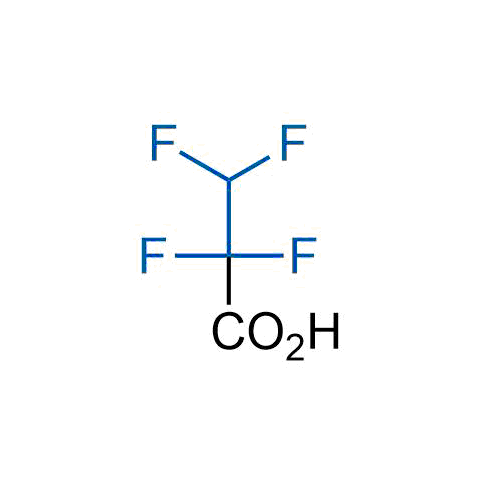
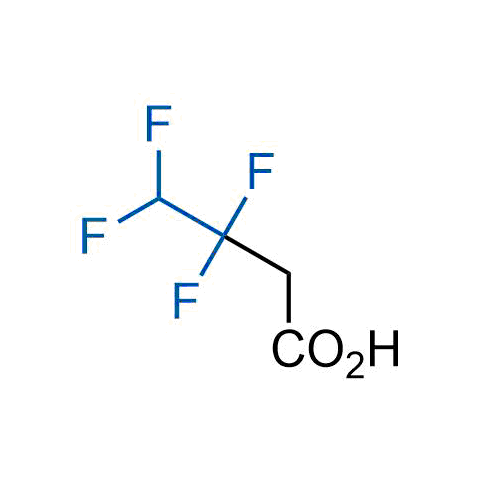
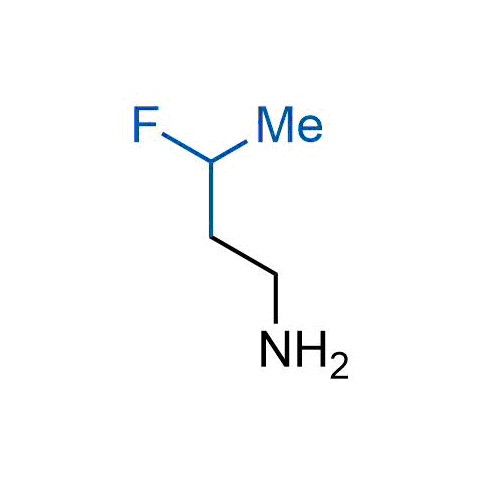
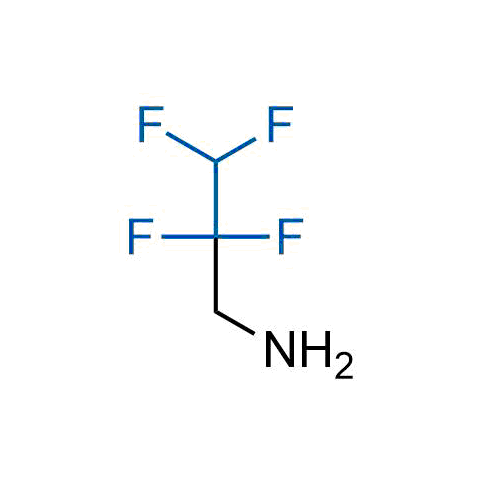
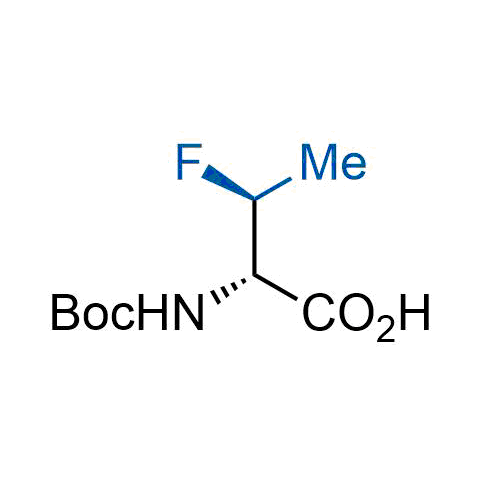
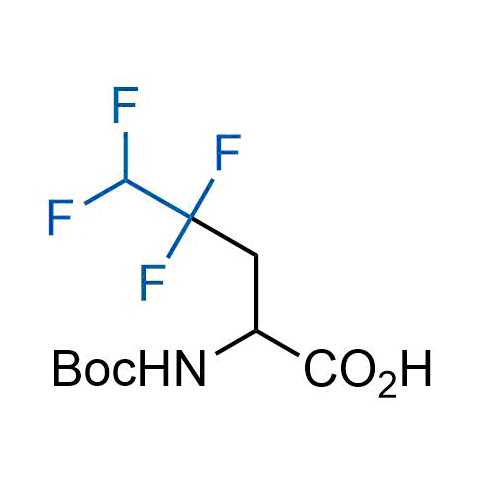
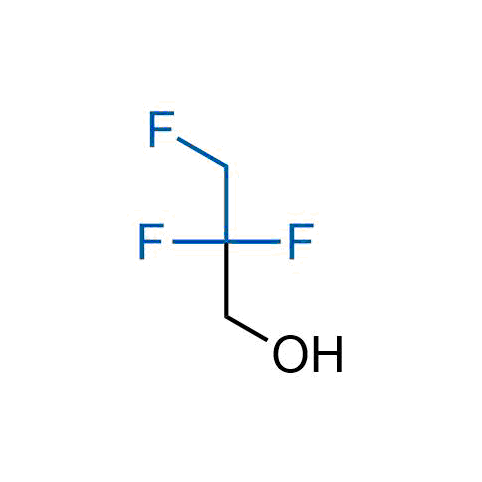
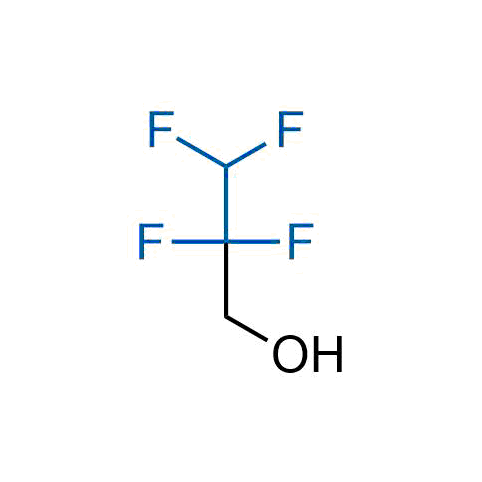
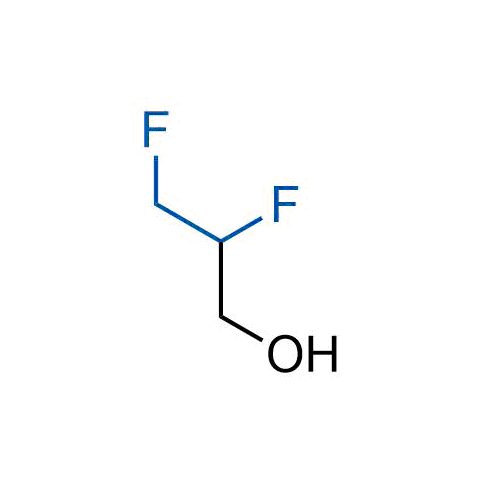
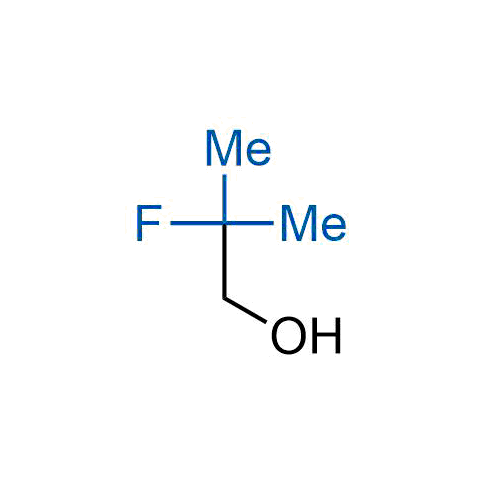
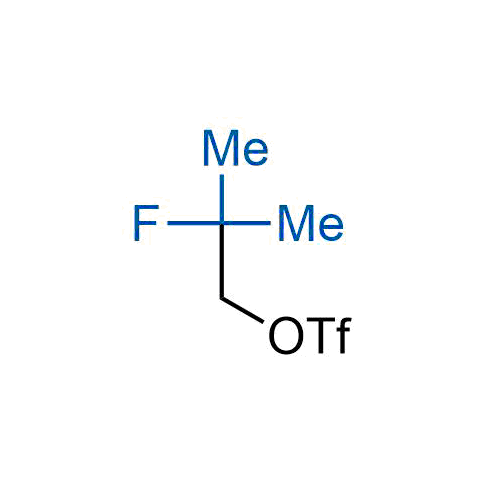
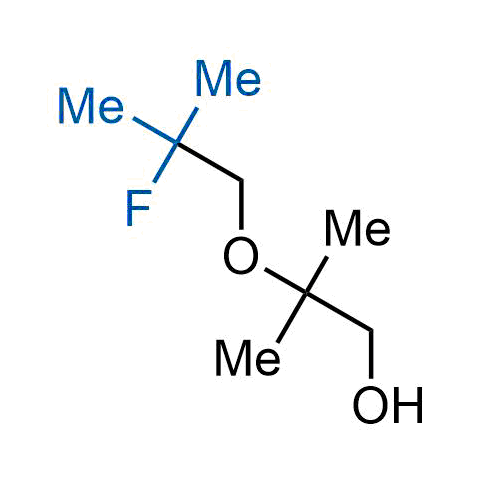
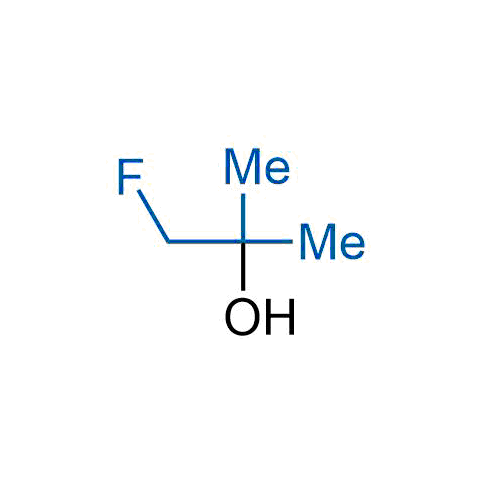
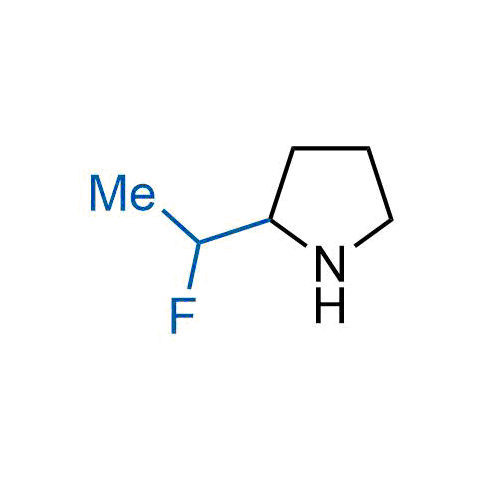
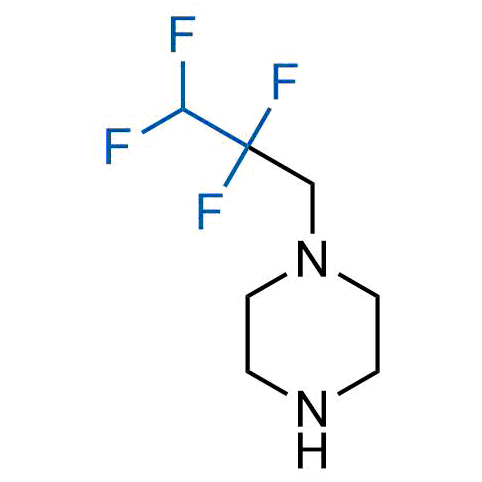
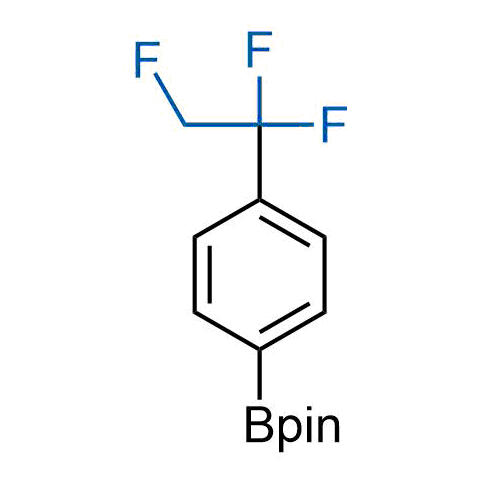
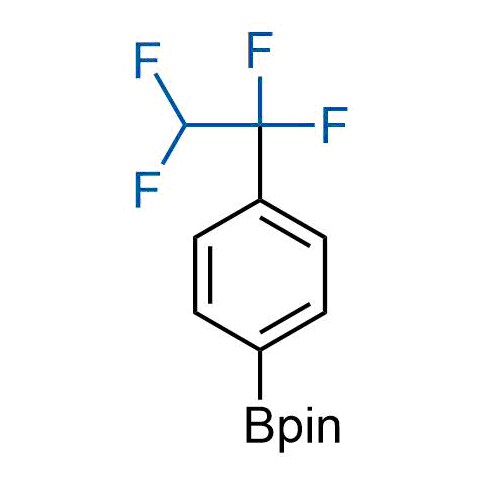
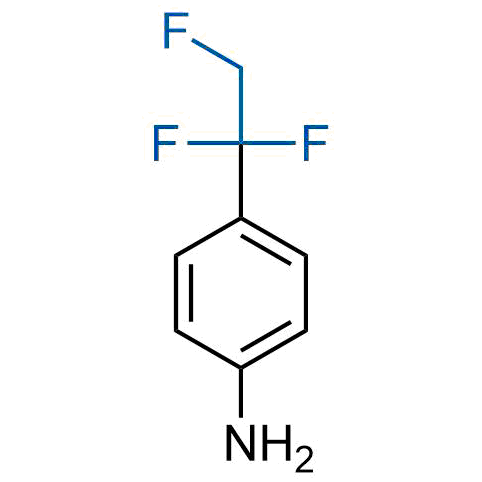
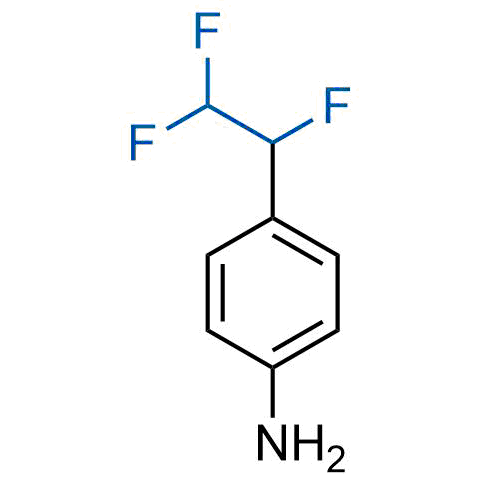
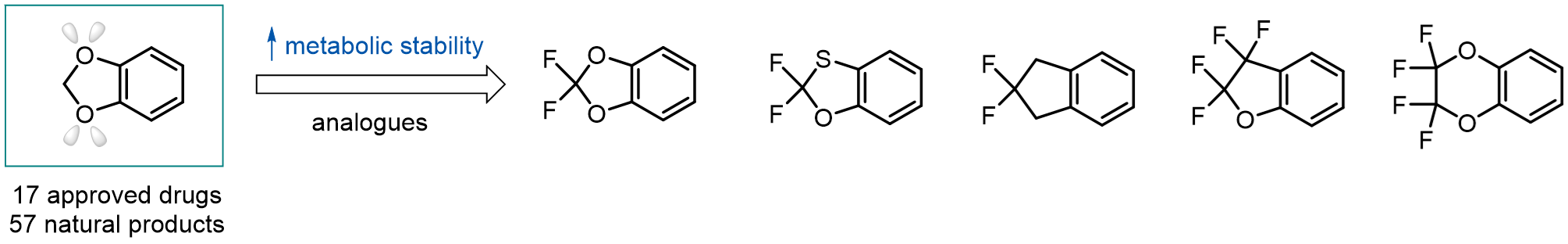
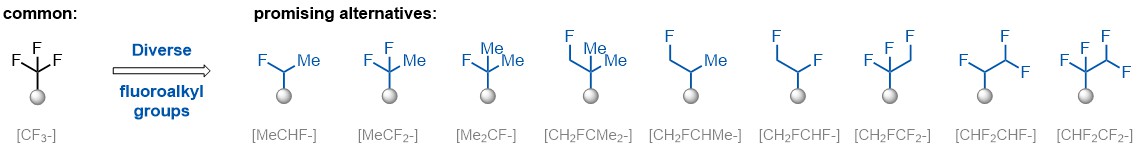
A wide variety of fluoroaliphatic moieties remain severely underexplored in drug design, in contrast to the CF3-group, which is among the most frequently found fragments in modern pharmaceuticals. In recent years, a growing set of examples have demonstrated a considerable potential of variable non-traditional fluoroalkyl groups in both, medicinal chemistry and agrochemical fields. These structures may address the issues associated with lipophilicity profile and metabolic degradation schemes of biologically active molecules.
Concept
We offer
More than 100 unique fluoroalkyl compounds from stock on a 5-10 g scale.
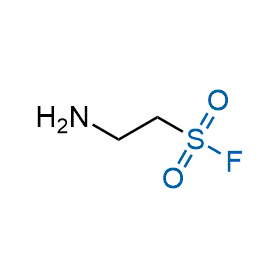
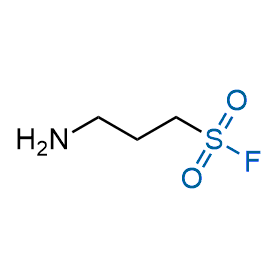
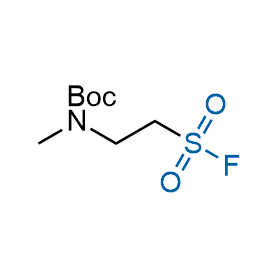
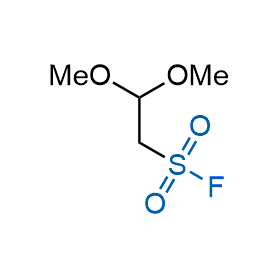
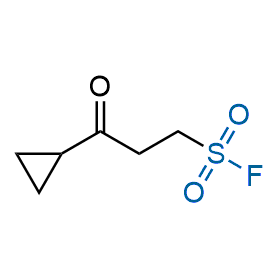
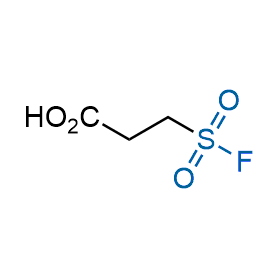
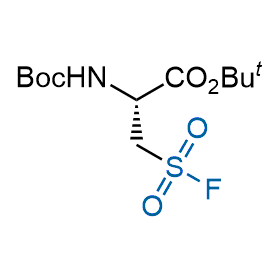
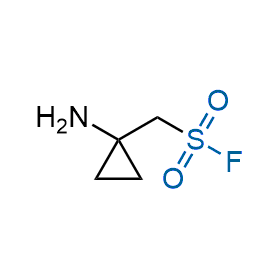
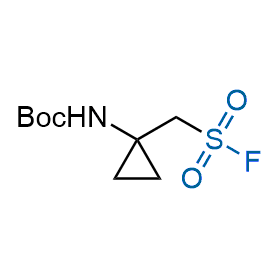
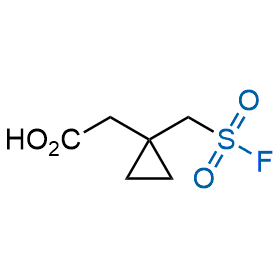
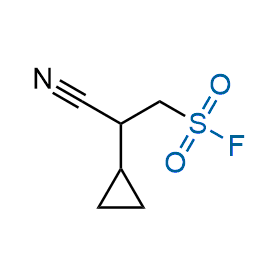
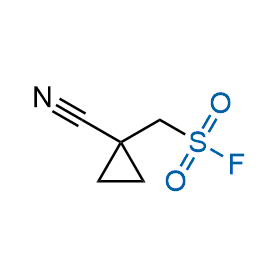
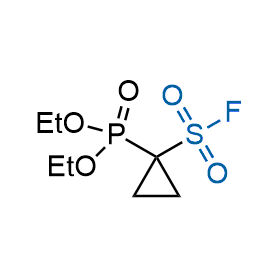
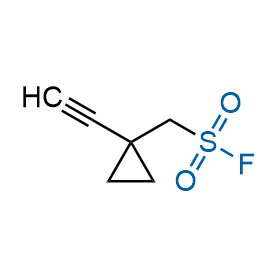
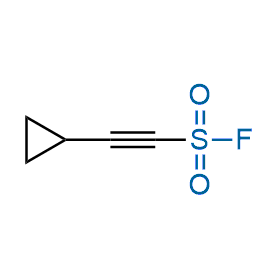
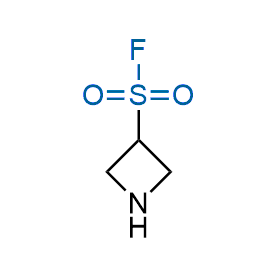
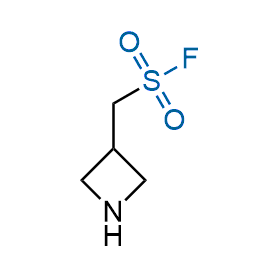
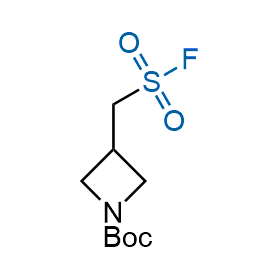
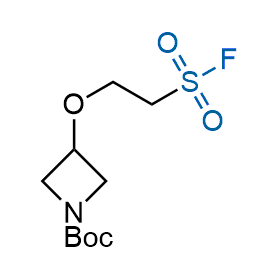
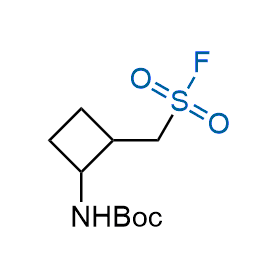
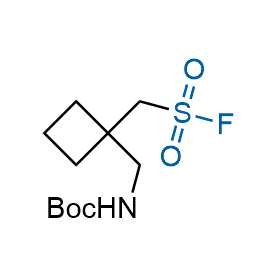
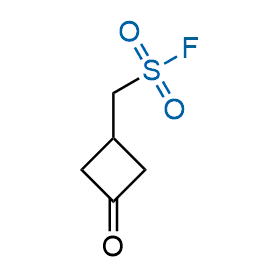
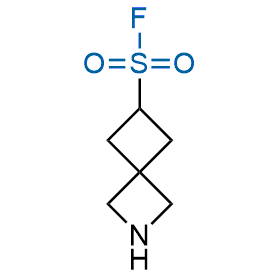
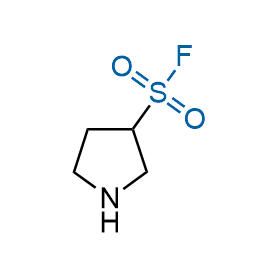
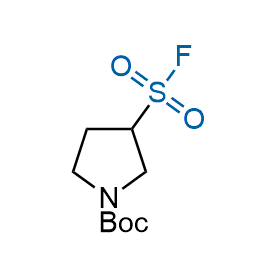
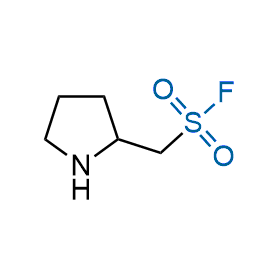
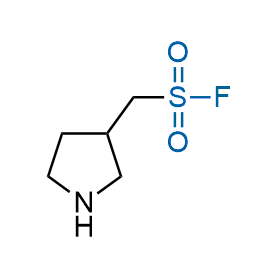
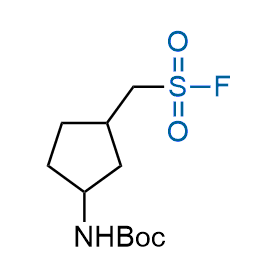
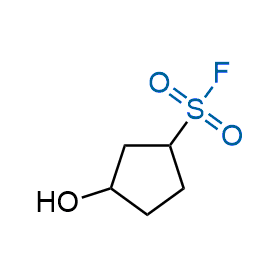
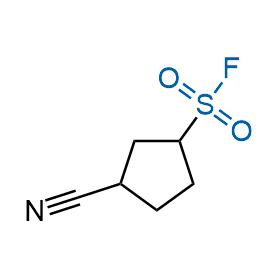
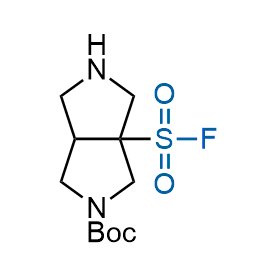
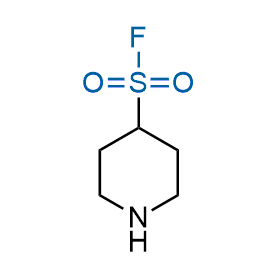
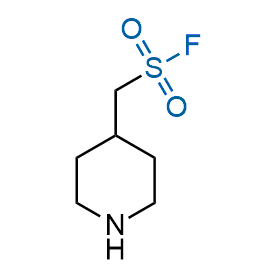
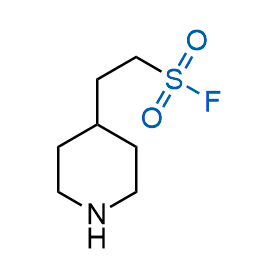
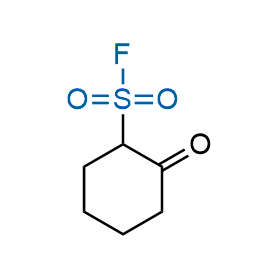
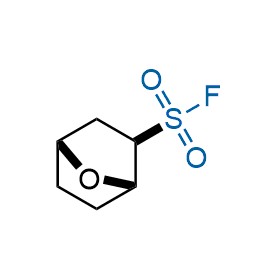
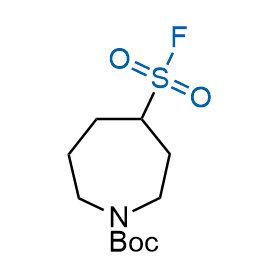
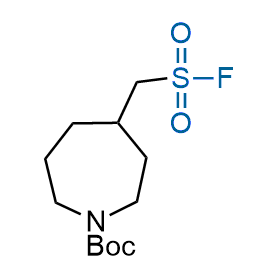
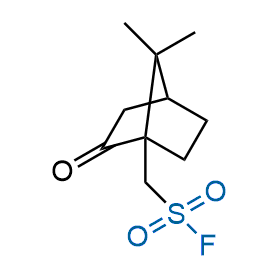
Following the original success of the alkyne-azide click chemistry, the sulfur (VI) fluoride exchange (SuFEx) has gained a wide recognition as a new family of click transformations. Aliphatic sulfonyl fluorides are excellent SuFEx agents owning to their moderate reactivity. These substances tolerate many other functional groups as well as handling in aqueous buffers. Yet, they react when attached to biomolecules, e.g., due to protein binding, forming strong covalent bonds. This mechanism makes them promising in designing irreversible protein inhibitors and protein labeling probes.
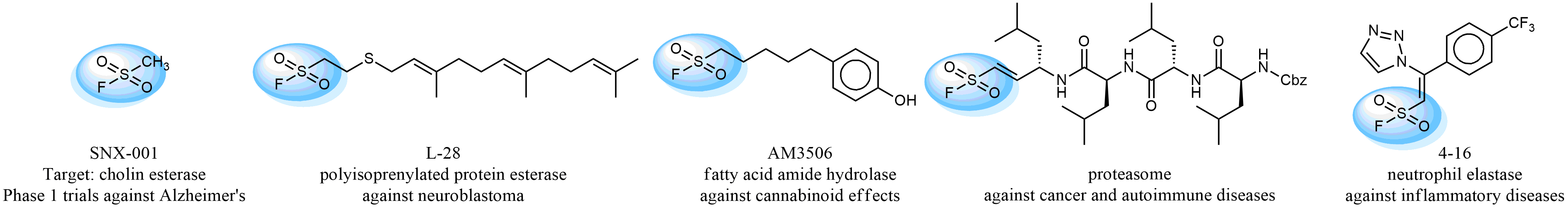
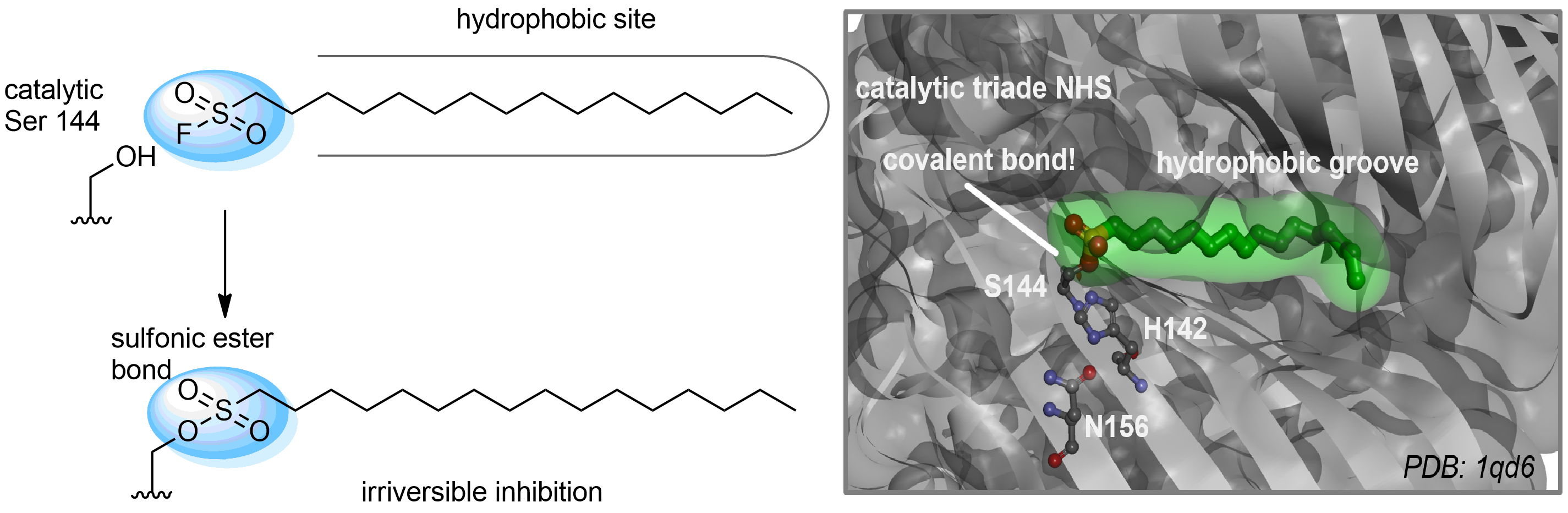
Case studies
Hexadecanesulfonyl fluoride (AM 374) binds to the hydrophobic groove of the inner interface formed by homodimer of outer membrane phospholipase A. Subsequent formation of a sulfonic ester with the catalytic serine-144 leads to irreversible inhibition.
Download SD file
Download PDF file
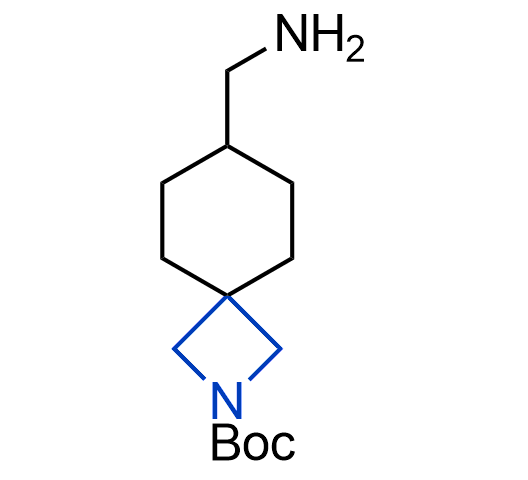
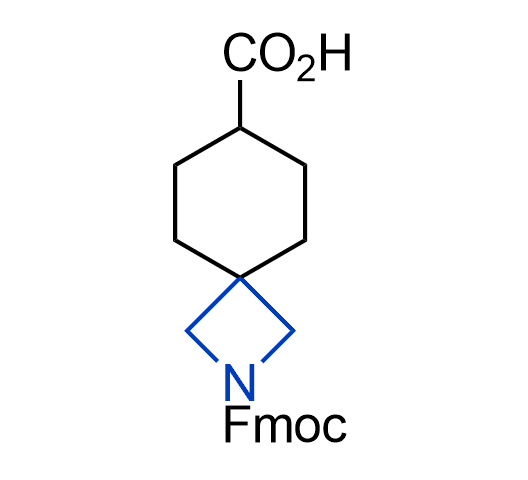
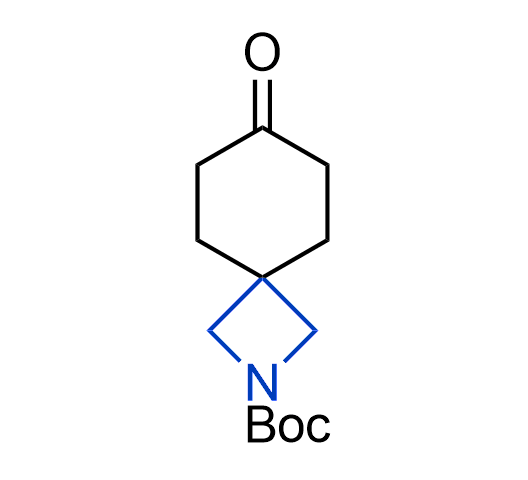
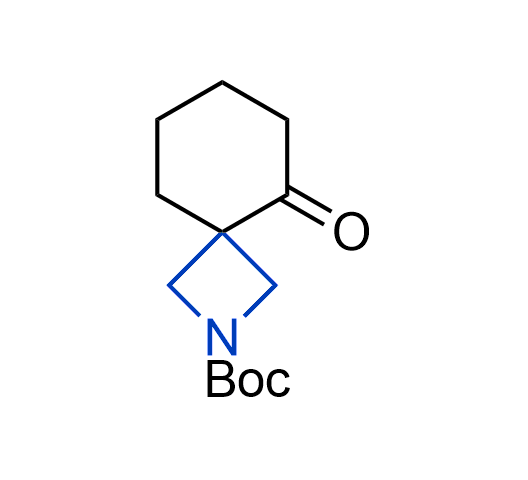
We offer
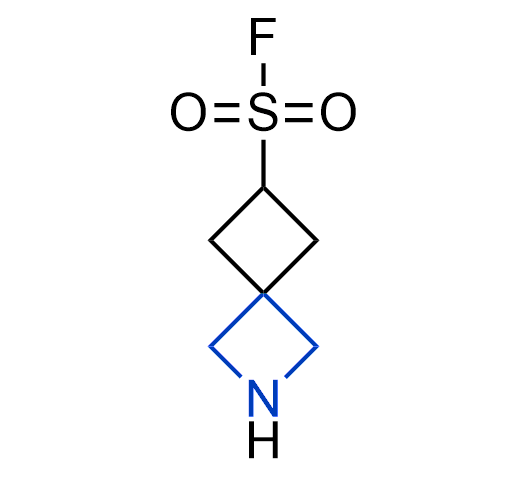
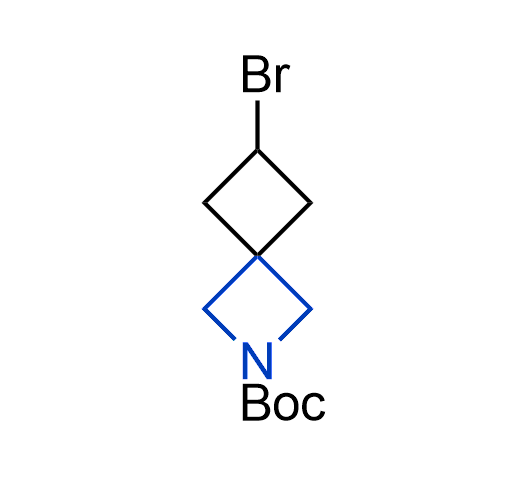
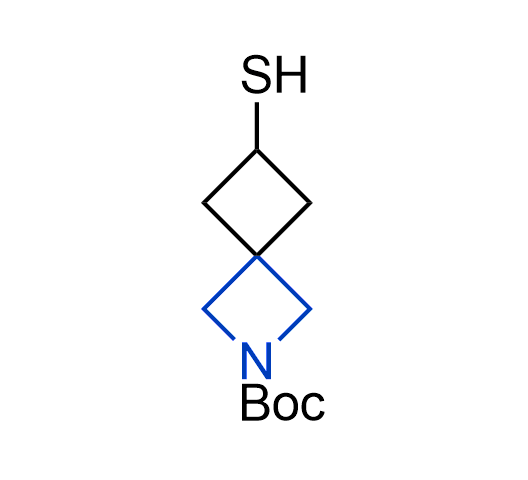
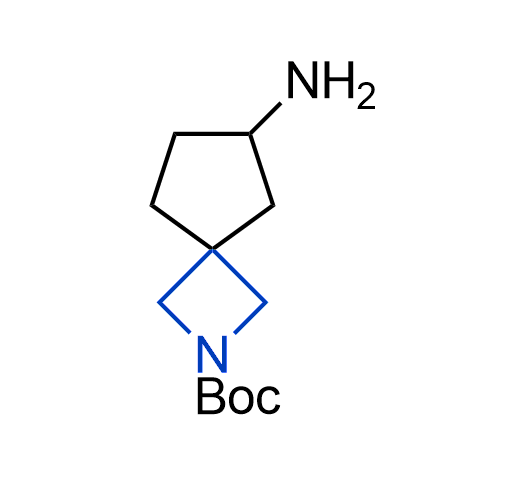
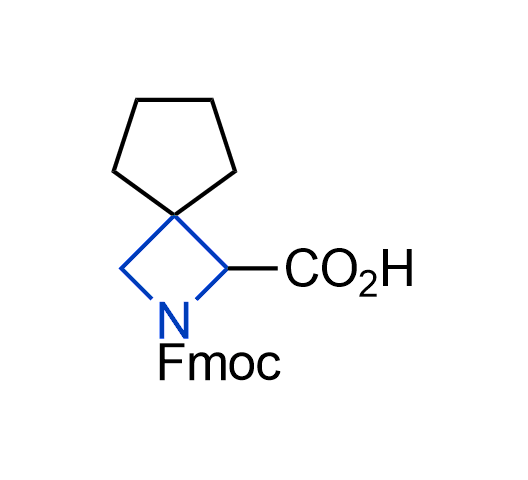
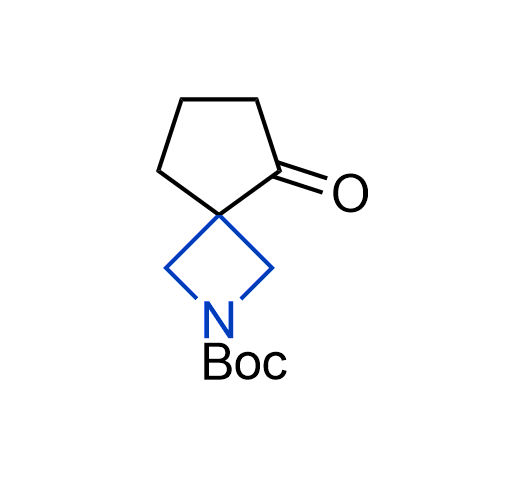
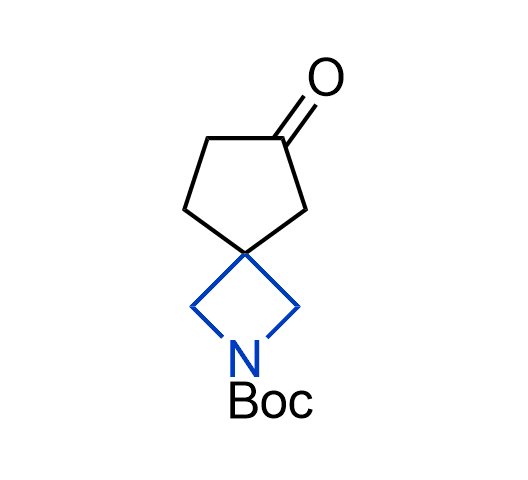
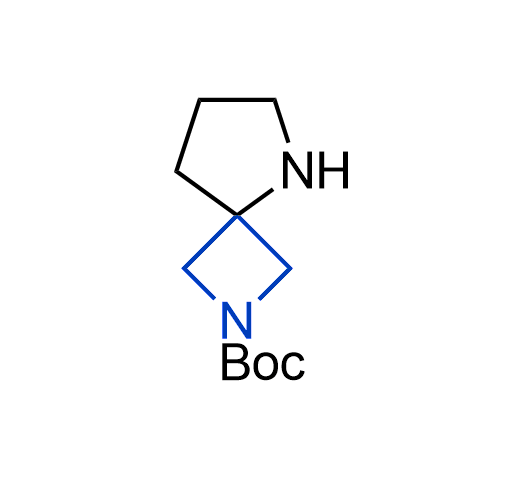
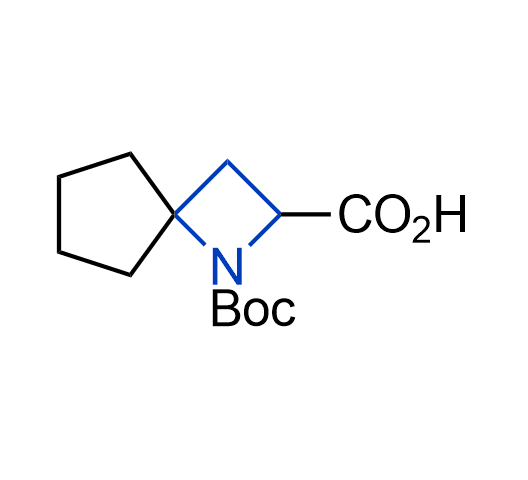
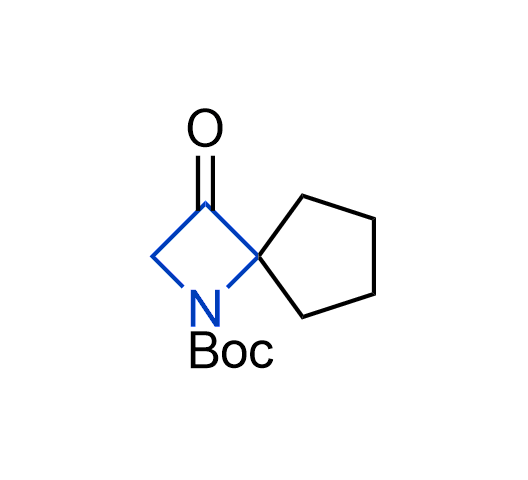
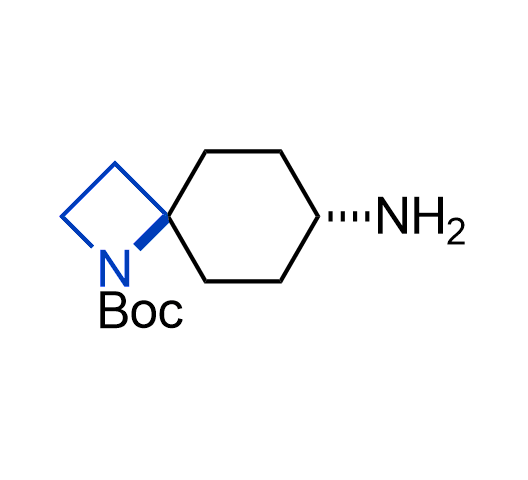
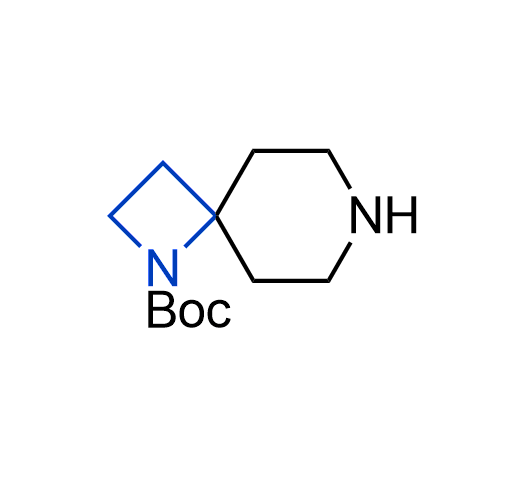
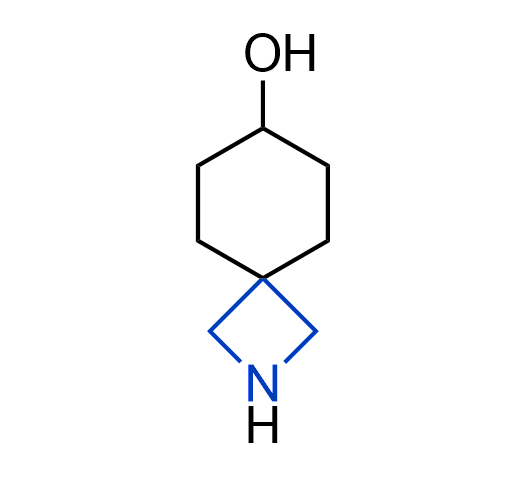
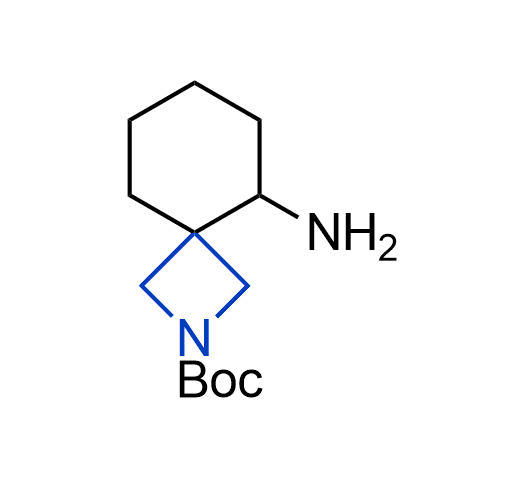
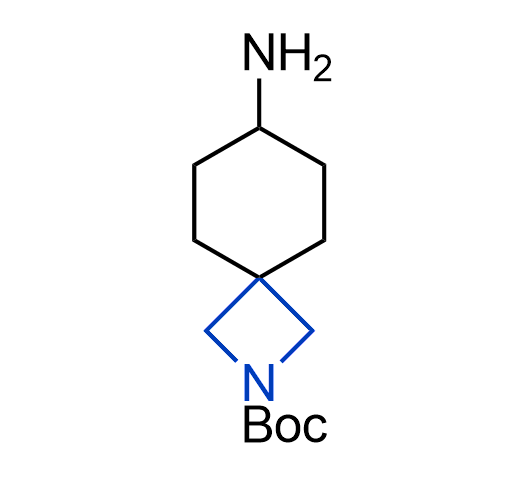
More than 100 of spirocyclic azetidines from stock on a 5-10 g scale.
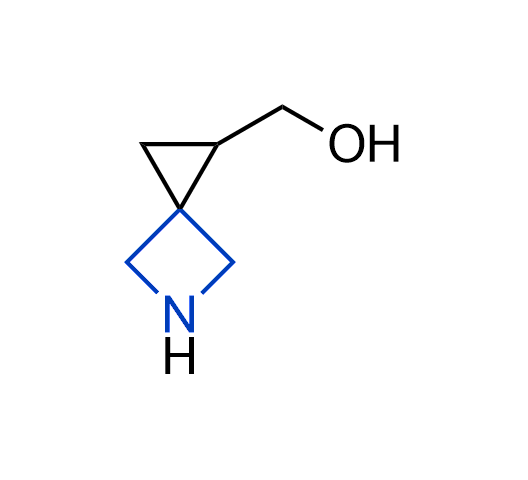
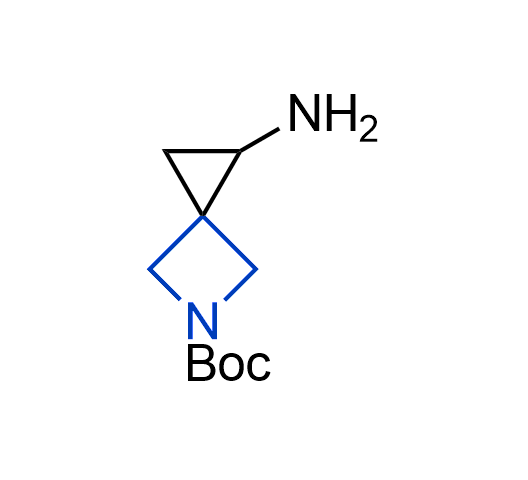
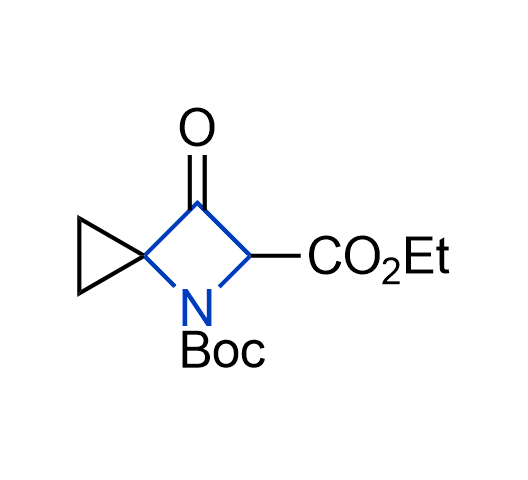
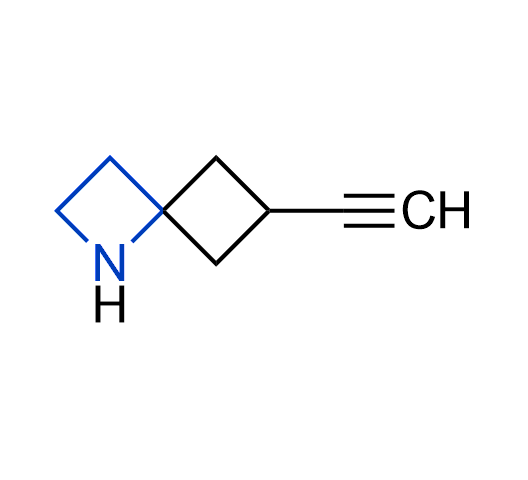
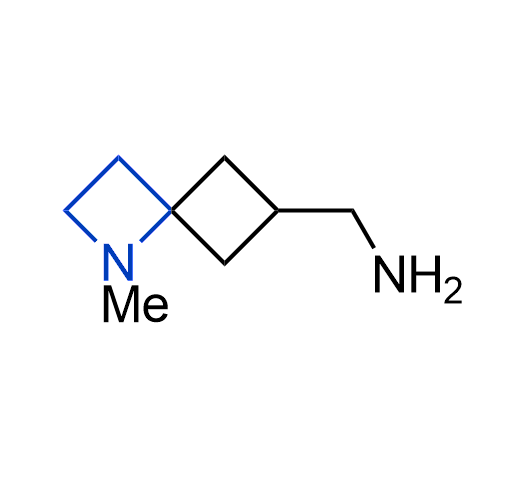
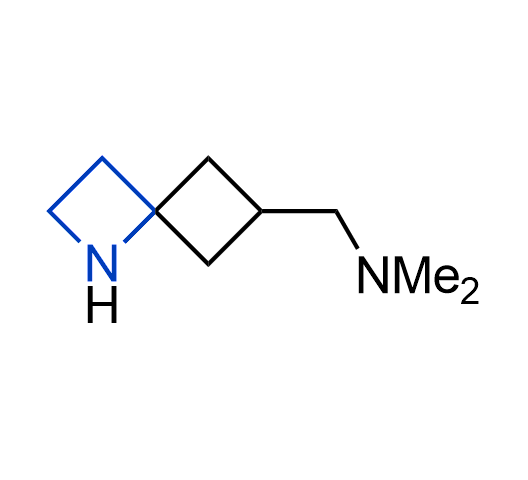
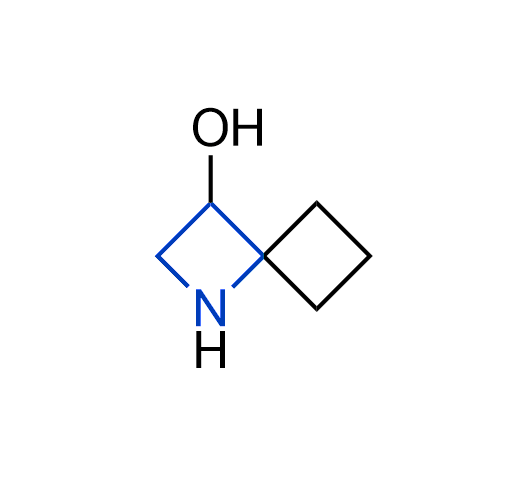
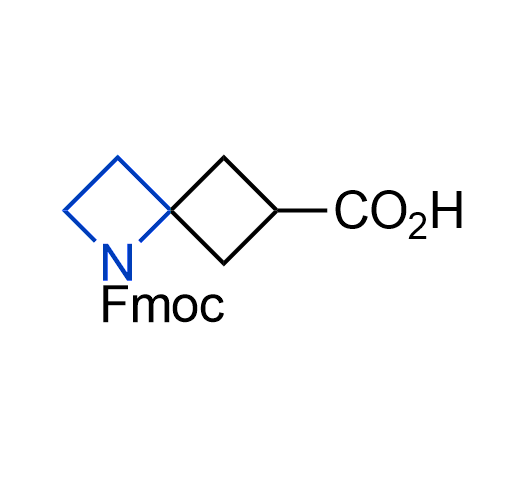
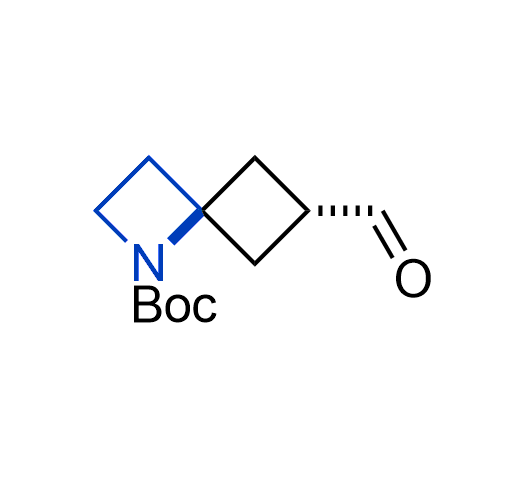
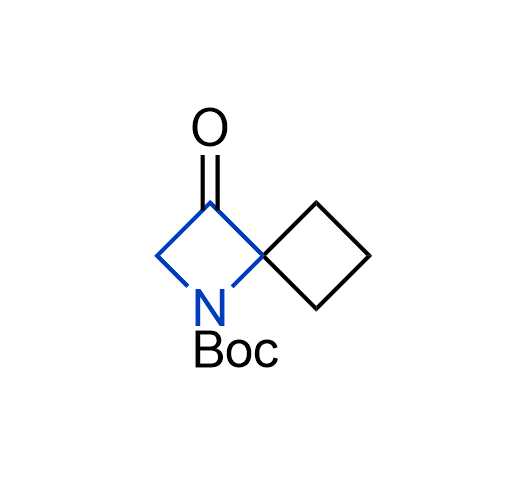
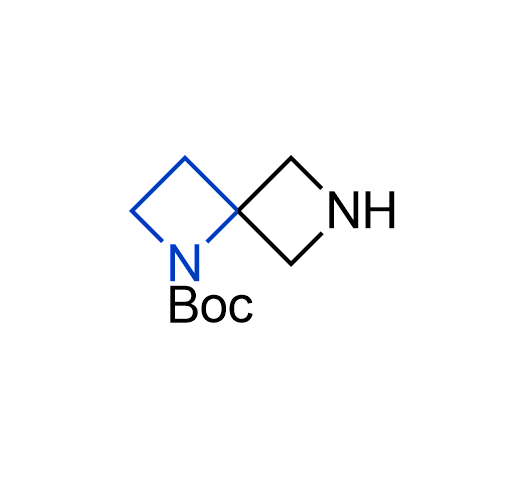
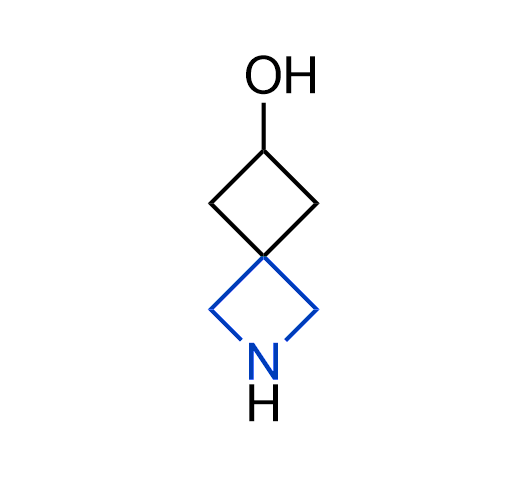
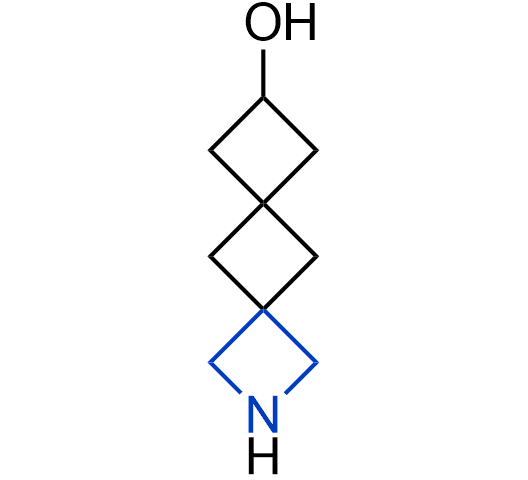
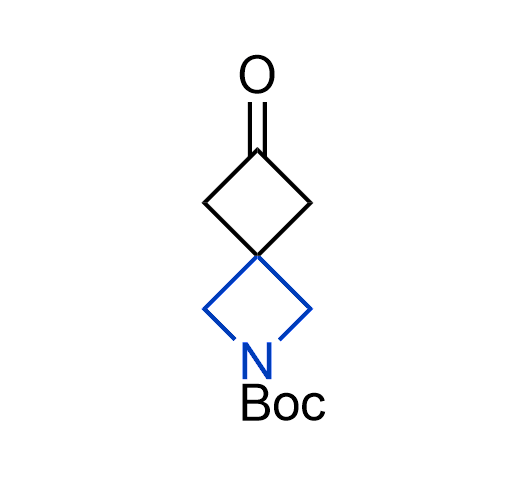
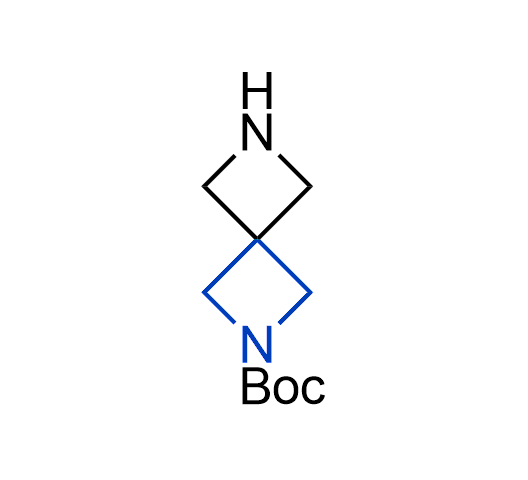
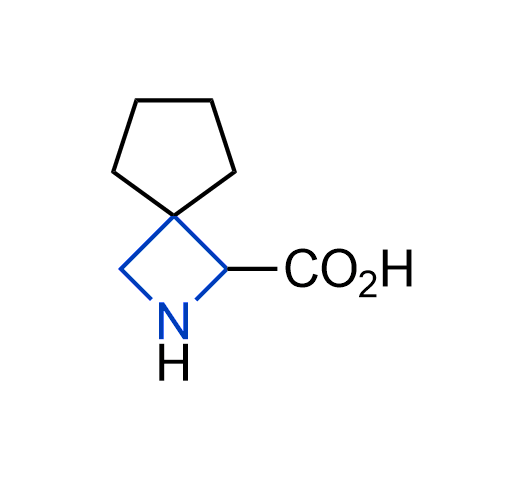
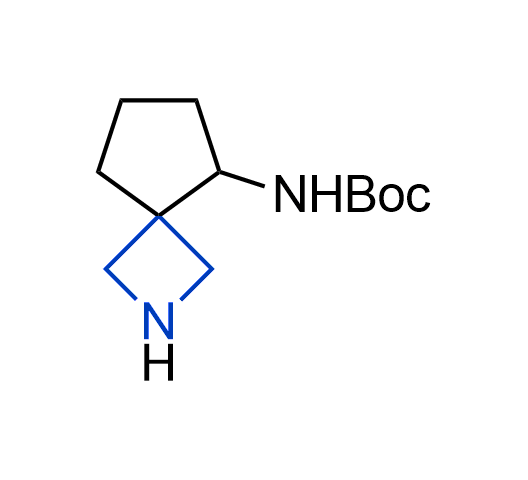
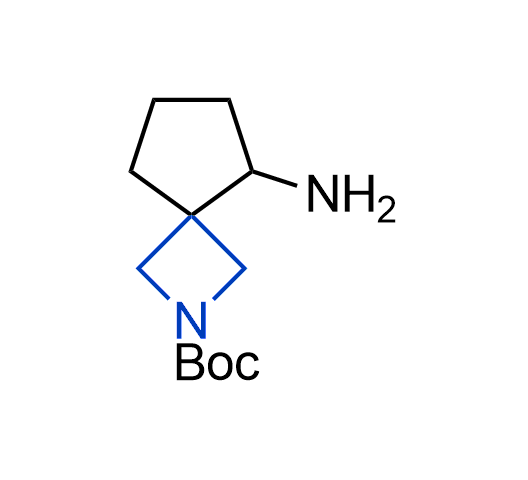
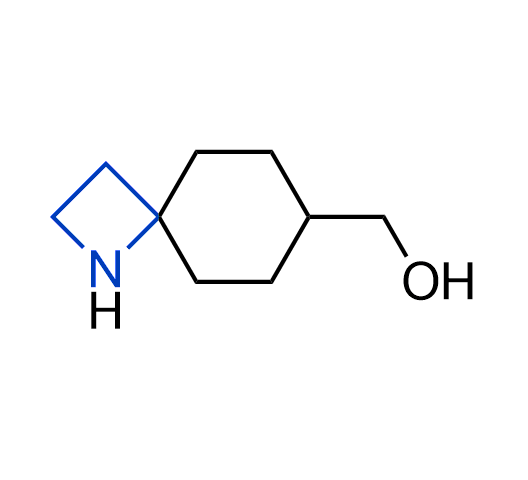
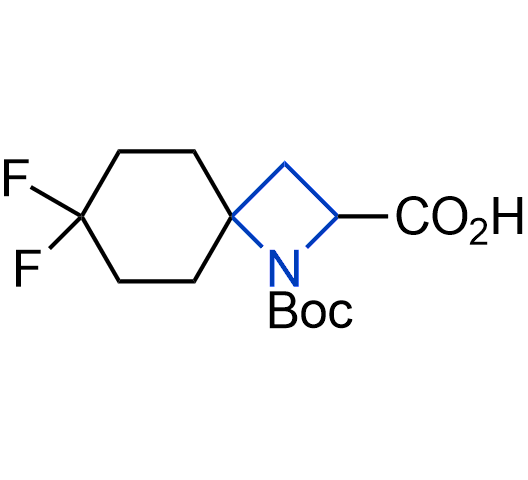
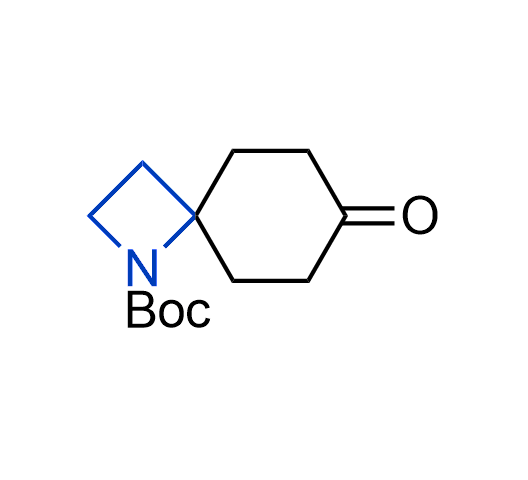
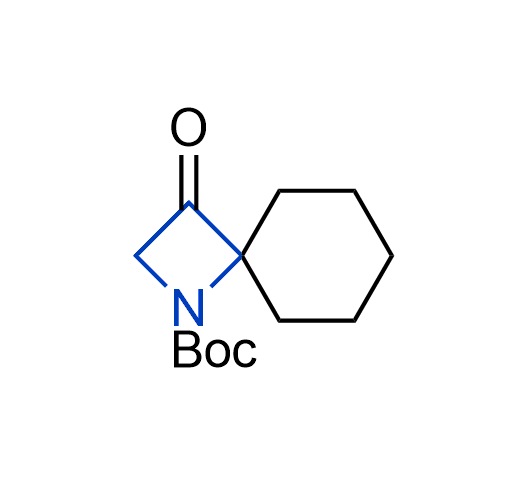
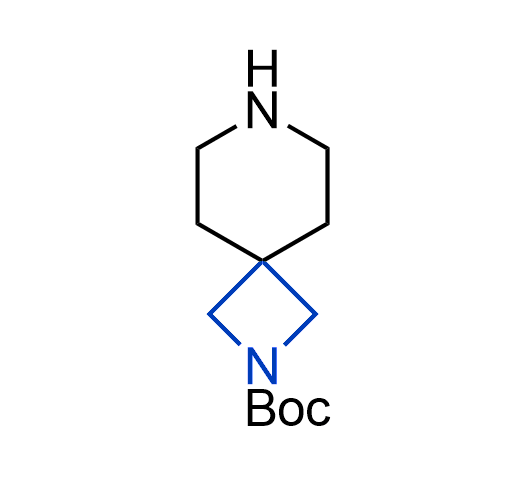
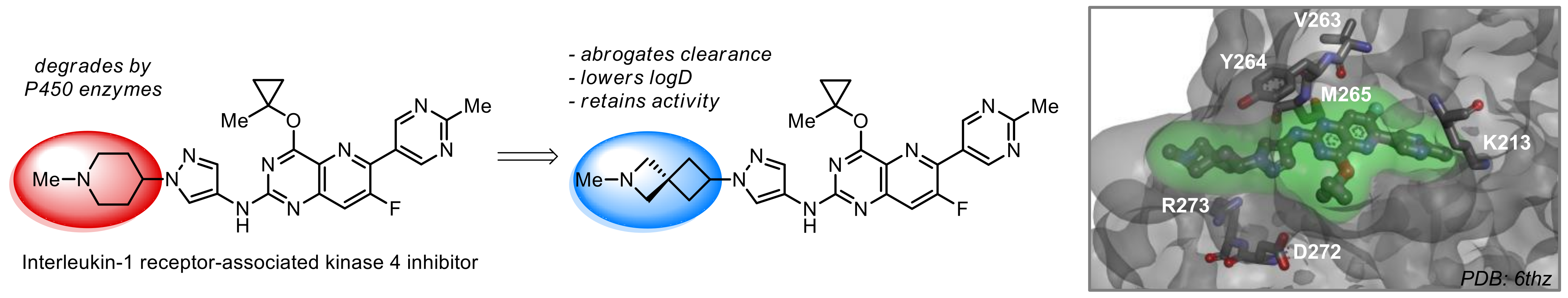
What if we could freely steer the geometry of lead compounds? With the ability to vary the ring fusion site, size of the rings, and the substitution patterns, this task is well addressed by spirocyclic azetidine structures. Furthermore, unlike analogous pyrrolidines or aromatic rings, spirocyclic azetidines are poorly recognized by degradation enzymes. Thus, introduction of spirocyclic azetidines has become an effective method for mitigating early drug clearance.
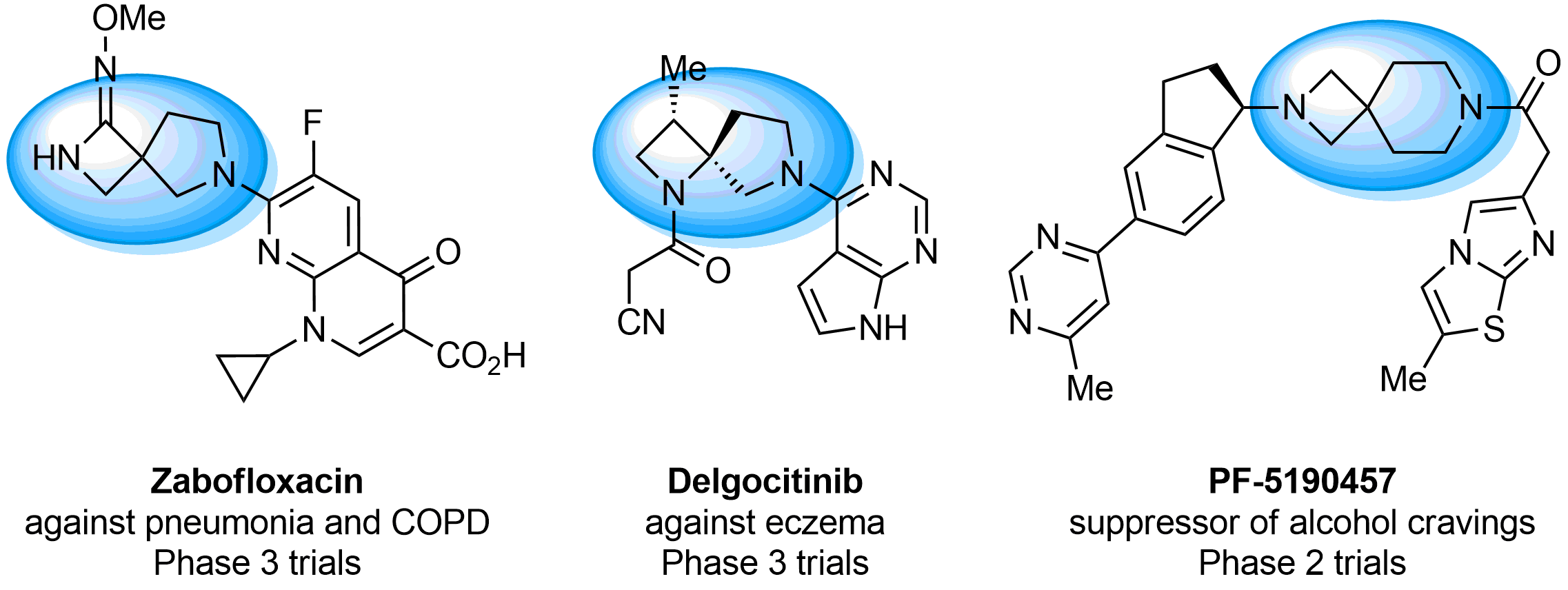
Case studies
Download SD file
Download PDF file
We offer
More than 100 of spirocyclic azetidines from stock on a 5-10 g scale.