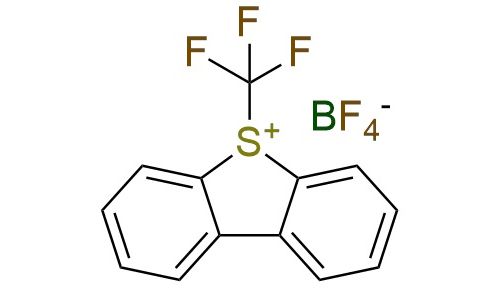
CAS 131880-16-5, Cat. No EN300-18527578
Reagent for trifluoromethylation
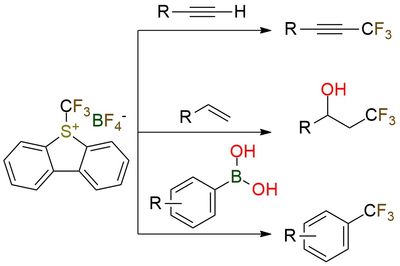
Umemoto reagent I is an electrophilic trifluoromethylating agent used for transferring the trifluoromethyl group to nucleophiles such as silyl enol ethers and β-ketoesters1. It is also employed in catalytic strategies including Pd-catalyzed aromatic C-H trifluoromethylation, Cu-catalyzed trifluoromethylation, and photoredox-catalyzed radical trifluoromethylation2. The reagent is a white crystalline solid that should be stored in a cool, dry place due to its potentially harmful properties.
Synonyms: S-(trifluoromethyl)dibenzothiophenium tetrafluoroborate; Umemoto’s reagent I; 5-(trifluoromethyl)-5H-dibenzo[b,d]thiophen-5-ium tetrafluoroborate; 5-(trifluoromethyl)dibenzothiophen-5-ium, tetrafluoroborate
Selected publications
-
Exploration of Fluorination Reagents Starting from FITS Reagents.
Umemoto T. J Fluor Chem 2014, 167, 3–15. DOI: 10.1016/j.jfluchem.2014.07.029
-
S-(Trifluoromethyl)Dibenzothiophenium Tetrafluoroborate.
Riou M.; Koike T. Encyclopedia of Reagents for Organic Synthesis 2018, 1–3. DOI: 10.1002/047084289X.rn01212.pub2