CAS 1034-39-5, Cat. No EN300-1664590
Reagent for bromination, cyclization, and dehydration
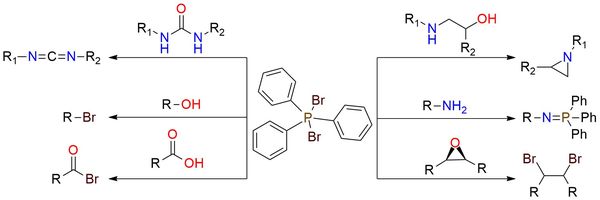
Triphenylphosphine dibromide is a reagent with many applications, primarily, converting alcohols, phenols, enols, ethers, and acetals into alkyl bromides, and carboxylic acid derivatives into acyl bromides1. This method can be applied to sensitive substrates that contain cyclopropyl rings (e.g., cyclopropyl carbinol) or unsaturated compounds (e.g., cinnamyl alcohol and alkynediols) with minimal or no side reactions. Additionally, the reagent facilitates the cyclization of amino alcohols to cyclic amines and can dehydrate and brominate carboxamide groups. Triphenylphosphine dibromide is a moisture-sensitive, hygroscopic solid with corrosive properties that require careful handling.
Synonyms: bromotriphenylphosphonium bromide; phosphorane, dibromotriphenyl-; dibromotriphenylphosphorane; dibromo(triphenyl)-lambda5-phosphane
Selected publication
-
Triphenylphosphine Dibromide.
Dormoy J.; Castro B.; Bobinski T. Encyclopedia of Reagents for Organic Synthesis 2018, 1–13. DOI: 10.1002/047084289X.rt370.pub2