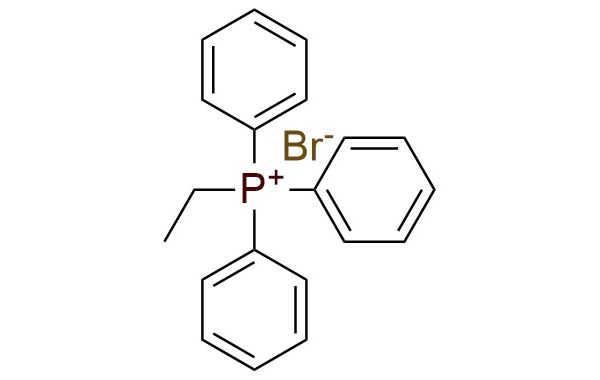
CAS 1530-32-1, Cat. No EN300-52977
Reagent for Wittig reaction
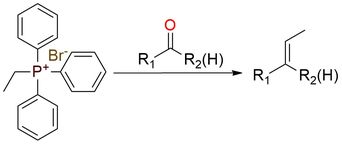
Triphenylethylphosphonium bromide (TEP) is a phosphonium salt that entails the transfer of an ethylidene group by generation of the ‘nonstabilized’ phosphorus ylide to aldehydes and ketones. It is a white to off-white solid, that is shelf-stable but is moister sensitive. The advantage of the synthetic approach is the fact that the stereoselectivity of the reaction with ylide is predictable and controllable, resulting in moderate to good yields1. This reagent can be used in the Schlosser modification of the Wittig reaction1.
Synonyms: ethyltriphenylphosphonium bromide (6CI, 7CI); phosphonium, ethyltriphenyl-, bromide (8CI, 9CI); TEP (onium compound); ethyltriphenylphosphanium bromide
Selected publication
-
Ethyltriphenylphosphonium Bromide.
McComsey D.; Maryanoff B. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.re123