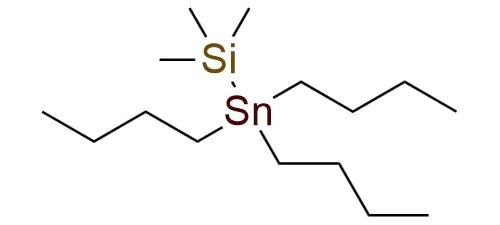
CAS 17955-46-3, Cat. No EN300-7404908
Organostannane reagent
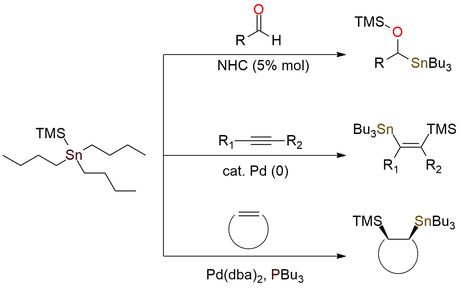
Trimethylsilyltributylstannane (Bu3SnTMS) is a popular choice in organostannane chemistry to embed the Bu3Sn group to a compound and as a bonus bears a very versatile TMS protection group1,2. The TMS group allows one to reach multiple synthetic tasks in a single pot. Bu3SnTMS is a colorless liquid soluble in THF, ether, DCM, MeOH, hexane, and insoluble in water1. It is moderate air- and moisture-stable and can be stored at room temperature for months. But, as with any tin chemistry, all precautions must be taken during the work1. The reagent is employed in reactions with aldehydes for synthesizing α-silyloxyalkylstannanes or γ-slyloxyallylstannanes1,2. It is a mild and convenient method, suitable for base-sensitive substrates. Besides this, the reagent is appropriate for addition to alkynes or alkenes due to its high tolerance to many functional groups. The applicable alkenes include ethylene, strained bicyclic alkenes, cyclopropenes, etc.1
Synonyms: (tributylstannyl)trimethylsilane; (trimethylsilyl)tributylstannane; (trimethylsilyl)tributyltin; tributyl(trimethylsilyl)tin; trimethyl(tributylstannyl)silane; Bu3SnTMS
Selected publications
-
Trimethylsilyltributylstannane.
RajanBabu T. (Babu); Shin S. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rn00647
-
N‐Heterocyclic Carbene‐Mediated Organocatalytic Transfer of Tin onto Aldehydes: New Access to α‐Silyloxyalkylstannanes and γ‐Silyloxyallylstannanes.
Blanc R.; Commeiras L.; Parrain J. Adv Synth Catal 2010, 352 (4), 661–666. DOI: 10.1002/adsc.200900816