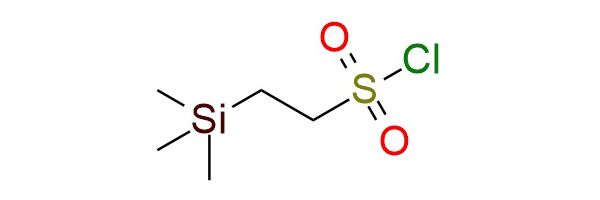
CAS 106018-85-3, Cat. No EN300-262942
Fluoride-cleavable N-protection
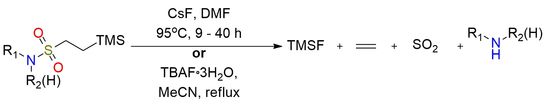
β-Trimethylsilylethanesulfonyl chloride (SES-Cl) is a versatile sulfonyl chloride reagent primarily used for amine protection as the SES sulfonamide1. The SES protecting group is uniquely valuable because, unlike typical sulfonamides (which are challenging to cleave), SES-sulfonamides are readily deprotected by fluoride sources (CsF, TBAF), producing the free amine under mild conditions. SES-Cl reacts smoothly with primary and secondary amines, as well as aromatic and heterocyclic amines, giving stable sulfonamides useful in multistep synthesis. The SES group is highly robust to acidic and Lewis acidic conditions, strong nucleophiles, and many oxidants/reductants, enabling transformations such as glycosylations, Diels–Alder reactions, addition of organometallic reagents to SES-imines, cycloadditions, and metal-catalyzed aziridination. SES derivatives also serve as intermediates to N-sulfinyl SES imines, key electrophiles for asymmetric synthesis, aza-Baylis–Hillman chemistry, and the construction of pyrrolines, pyrroles, and heterocycles. The reagent is a stable, distillable liquid that is soluble in common organic solvents but prone to hydrolysis.
Synonyms: SES-Cl; 2-(trimethylsilyl)ethane-1-sulfonyl chloride; 2-trimethylsilanyl-ethanesulfonyl chloride; beta-trimethylsilylethanesulfonyl chloride
Selected publication
-
β-Trimethylsilylethanesulfonyl Chloride.
Weinreb, S. M.; Ralbovsky, J. L.; Declerck, V.; Ribière, P.; Martinez, J.; Lamaty, F. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rt300.pub2