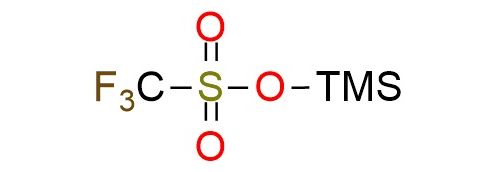
CAS 27607-77-8, Cat. No EN300-37509
Reagent for silylation
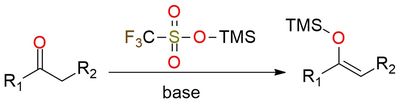
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) is a highly electrophilic silylating reagent used extensively in organic synthesis for O- and C-silylation reactions1. Its scope includes converting carbonyl compounds to enol ethers and facilitating reactions such as aldol-type condensations, glycosidations, and cyclizations. The reagent is utilized for the protection of hydroxyl groups and for promoting transformations that require activation of carbonyl and other electrophilic groups, making it essential for preparing enol ethers, silyl enolates, and silyl iminium ions. It is a colorless, moisture-sensitive, flammable, and corrosive liquid, necessitating careful handling and storage under inert, dry conditions.
Synonyms: trimethylsilyl trifluoromethanesulfonate; TMSOTf; trimethylsilyltrifluoromethanesulfonate; trifluoromethanesulfonic acid trimethylsilyl ester; trimethylsilyl trifluoromethylsulfonate; trimethylsilyl trifluoromethylsulphonate; trimethylsilyl trifluoromethanesulphonate; methanesulfonic acid, trifluoro-, trimethylsilyl ester
Selected publication
-
Trimethylsilyl Trifluoromethanesulfonate.
Sweeney J.; Perkins G.; Aguilar E.; Fernández-Rodríguez M.; Marquez R.; Amigues E.; Lopez-Gonzalez R. Encyclopedia of Reagents for Organic Synthesis 2018, 1–36. DOI: 10.1002/047084289X.rt338.pub3