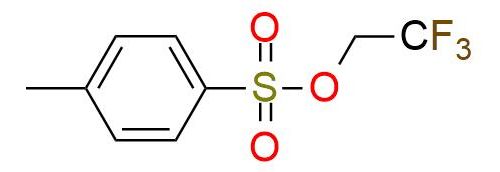
CAS 433-06-7, Cat. No EN300-30885
Reagent for trifluoroethylation
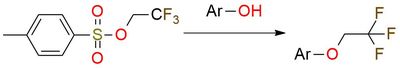
Trifluoroethanol tosylate is a widely used reagent for the efficient etherification of aromatic alcohols with the -CH2CF3 group. It is a solid moisture-sensitive white powder with a relatively low melting point1. The reagent is selective to aromatic alcohols and ignores other functional groups like carbonyl2. The etherification process with this reagent is routine and has a good yield. Additionally, trifluoroethanol tosylate is a valuable precursor for essential building blocks used in the difluoroethenylation of arenes through coupling reaction2.
Synonyms: 2,2,2-trifluoroethyl 4-methylbenzenesulfonate; 2,2,2-trifluoroethyl tosylate; trifluoroethanol tosylated; 2,2,2-trifluoroethyl 4-methylbenzene-1-sulfonate; trifluoroethanol tosylated; 2,2,2-trifluoroethyl p-toluenesulfonate
Selected publications
-
2,2,2-Trifluoroethyl p-Toluenesulfonate.
Ichikawa J. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rt243
-
A Simple Reagent for the Difluoroethenylation of Arenes.
Synfacts 2014, 10 (02), 0153–0153. DOI: 10.1055/s-0033-1340603