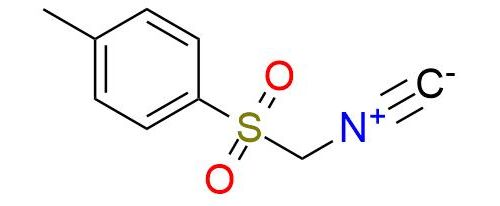
CAS 36635-61-7, Cat. No EN300-36947
Reagent for cyanation of carbonyl compounds and N-heterocycles synthesis
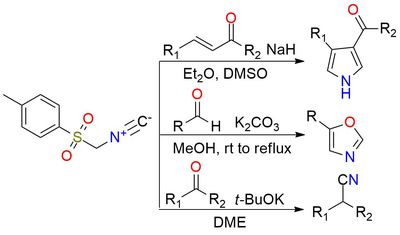
p-Tolylsulfonylmethyl isocyanide (TosMIC) is a unique and versatile reagent used in Van Leusen reaction and heterocyclic formations1. It is a colorless stable solid. Unlike many isocyanides, TosMIC is odorless. Reductive cyanation of ketones and aldehydes with TosMIC is a reliable reaction with excellent yields that can be performed with hindered substrates. Beyond the Van Leusen reaction, TosMIC plays an important role in forming heterocyclic compounds2. It provides a straightforward approach to synthesizing a wide range of azoles, including pyrroles, oxazoles, imidazoles, thiazoles, etc.
Synonyms: tosylmethyl isocyanide; (4-methylphenylsulfonyl)methyl isocyanide; (p-tolylsulfonyl)methyl isocyanide; 1-(isocyanomethanesulfonyl)-4-methylbenzene; 1-methyl-4-(((isocyano)methyl)sulfonyl)benzene; 4-toluenesulfonylmethyl isocyanide; 4-tolylsulfonylmethyl isocyanide; isocyanomethyl p-tolyl sulfone
Selected publications
-
P-Tolylsulfonylmethyl Isocyanide.
van Leusen A.; van Leusen D.; Czakó B. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rt150.pub2
-
Copper- or Phosphine-Catalyzed Reaction of Alkynes with Isocyanides. Regioselective Synthesis of Substituted Pyrroles Controlled by the Catalyst.
Kamijo S.; Kanazawa C.; Yamamoto Y. J Am Chem Soc 2005, 127 (25), 9260–9266. DOI: 10.1021/ja051875m