CAS 887144-97-0, Cat. No EN300-195971
Reagent for trifluoromethylation
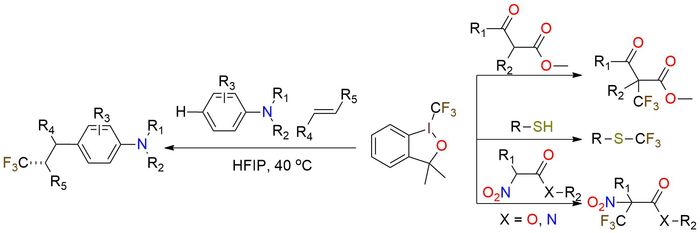
Trifluoromethyl-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole (Togni reagent) is used for direct electrophilic trifluoromethylation of various nucleophiles1,2. It is a white solid, soluble in most organic solvents, with properties similar to the Togni II reagent2. The reagent is also capable of selective reaction with thiols to yield corresponding trifluoromethyl thioethers. Togni reagent is less reactive and offers a softer approach. It has unique properties and is mainly used in reactions with β-keto esters or α-nitro carbonyl compounds1,3. Recently was developed a methodology for trifluoromethylarylation of alkenes with anilines and the Togni reagent, which offers good yields and utilizes no additives, transition metals, photocatalysts, or an excess of reagents2. Using this methodology, the Togni II reagent doesn’t react well or give low yields.
Synonyms: trifluoromethyl-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole; 3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole (ACI); 1-trifluoromethyl-3,3-dimethyl-1,2-benziodoxole; 3,3-dimethyl-1-trifluoromethyl-1,2-benzoiodoxole; Togni's Reagent
Selected publications
-
Novel 10‐I‐3 Hypervalent Iodine‐Based Compounds for Electrophilic Trifluoromethylation.
Eisenberger P.; Gischig S.; Togni A. Chemistry – A European Journal 2006, 12 (9), 2579–2586. DOI: 10.1002/chem.200501052
-
Trifluoromethyl-1,3-Dihydro-3,3-Dimethyl-1,2-Benziodoxole.
Stanek K.; Koller R.; Kieltsch I.; Eisenberger P.; Togni A. Encyclopedia of Reagents for Organic Synthesis 2009. DOI: 10.1002/047084289X.rn01120
-
Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents.
Charpentier J.; Früh N.; Togni A. Chem Rev 2015, 115 (2), 650–682. DOI: 10.1021/cr500223h