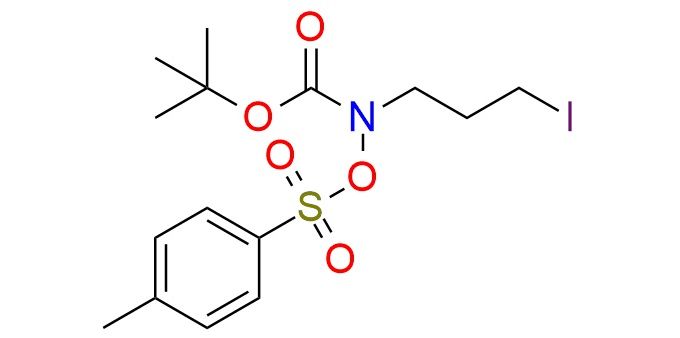
CAS 3087757-18-1, Cat. No EN300-53768872
Reagent for [C,N] annulation
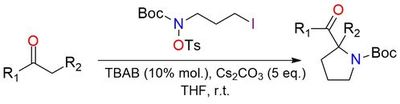
tert-Butyl (3-iodopropyl)(tosyloxy)carbamate is a highly tuned bifunctional [C,N] electrophile enabling umpolung (4+1) annulation with diverse carbonyl nucleophiles. It offers the best balance of stability and reactivity among tested analogues1, cleanly undergoing initial C-alkylation followed by SN2 C–N ring closure to form 2,2-disubstituted pyrrolidines in excellent yields across a wide range of β-keto esters, diketones, malonates, cyanoacetates, β-keto phosphonates, Meldrum’s acid, oxindoles, ketones, and non-stabilized enolates. It is stable under Cs₂CO₃/TBAB yet reactive enough to promote high-yield annulation, making it the key synthon of the methodology.
Synonyms: tert-butyl N-(3-iodopropyl)-N-[(4-methylbenzenesulfonyl)oxy]carbamate; tert-butyl (3-iodopropyl)(tosyloxy)carbamate; benzenesulfonic acid, 4-methyl-,[(1,1-dimethylethoxy)carbonyl](3-iodopropyl)azanyl ester; t-Bu (3-iodopropyl)(tosyloxy)carbamate
Selected publication
-
Umpolung (4+1) Annulations of Bifunctional Hydroxylamines: A Modular Approach to Pyrrolidines.
Zhao, Y.; He, W.; Xu, J.; Wang, J.; Gan, L.; Hu, L. Org Lett 2025, 27 (20), 5146–5151. DOI: 10.1021/acs.orglett.5c01285