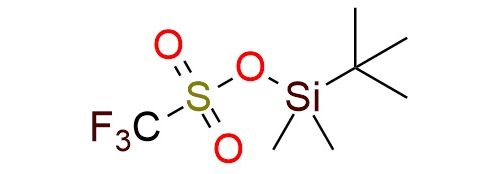
CAS 69739-34-0, Cat. No EN300-128285
Reagent for silylation
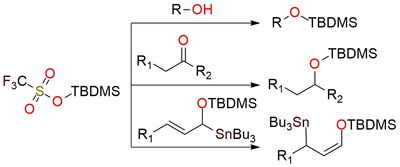
TBDMS triflate is a highly reactive silylating reagent and a Lewis acid1. The reagent's scope includes the conversion of primary, secondary, and tertiary alcohols to their corresponding TBDMS ethers, as well as the formation of enol silyl ethers from ketones and lactones. TBDMS triflate can promote conjugate additions, activation of carbonyl compounds, and rearrangement reactions. It catalyzes glycosylation and epoxide opening reactions and acts as a reagent in the stereoselective formation of cyclic acetals and oxazolidinones. The substance is a liquid, highly reactive with water, and should be stored under inert conditions (argon) at low temperatures (0°C). It reacts rapidly with protic solvents and is generally handled in non-aqueous conditions.
Synonyms: tert-butyldimethylsilyl trifluoromethanesulfonate; trifluoromethanesulfonic acid tert-butyldimethylsilyl ester; t-butyldimethylsilyl triflate; tert-butyldimethylsilyl trifluoromethanesulphonate; tert-butyldimethylsilyl triflate; TBDMS-OTf; TBDMS triflate; [tert-butyl(dimethyl)silyl] trifluoromethanesulfonate
Selected publication
-
Tert-Butyldimethylsilyl Trifluoromethanesulfonate.
Hua D.; Chen J.; Grainger R.; Aricó C. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rb381.pub2