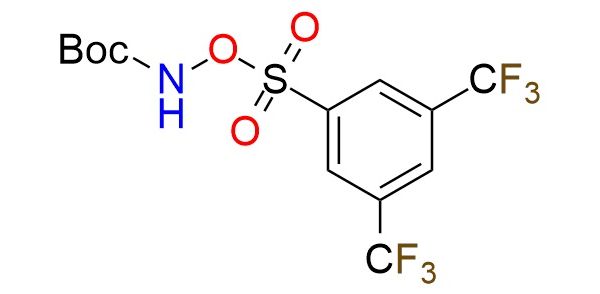
CAS 3079427-82-7, Cat. No EN300-52272785
Reagent for heterocycles skeletal editing
![t-Butyl N-{[3,5-bis(trifluoromethyl)benzenesulfonyl]oxy}carbamate](/images/Reagents/EN300-52272785_Scheme.jpg)
t-Butyl N-{[3,5-bis(trifluoromethyl)benzenesulfonyl]oxy}carbamate is a novel reagent used for the regiocontrolled synthesis of polysubstituted N-alkylpyrazoles through skeletal single atom editing1. The transformation starts with N-amination of isothiazoles, enabling the synthesis of 1,2,3-thiadiazine-S-oxides (TDSOs)1. These intermediates undergo ring expansion and SO extrusion to afford unsymmetrical N-alkyl pyrazoles with high regioselectivity. The reagent generates an electrophilic NH2+ species in situ, making it effective even with poorly nucleophilic substrates. It is bench-stable, scalable, and synthesized efficiently on a 100 mmol scale. This approach provides a powerful strategy for regiocontrolled pyrazole synthesis and the selective late-stage functionalization of heterocycles.
Synonyms: tert‐butyl N‐{[3,5‐bis(trifluoromethyl)benzenesulfonyl]oxy}carbamate; tert-butyl N-((3,5-bis(trifluoromethyl)phenyl)sulfonyl)oxycarbamate; N-Boc-O-(3,5-bistrifluoromethylphenyl)sulfonyl-hydroxylamine
Selected publication
-
Strategic Atom Replacement Enables Regiocontrol in Pyrazole Alkylation.
Le Xander Fanourakis A.; Ali Y.; Chen L.; Kelly P. Q.; Bracken A. J.; Kelly C. B.; Levin M. D. Nature 2025. DOI: 10.1038/s41586-025-08951-x