CAS 81037-06-1, Cat. No EN300-24469793
Reagent-precursor in asymmetric synthesis
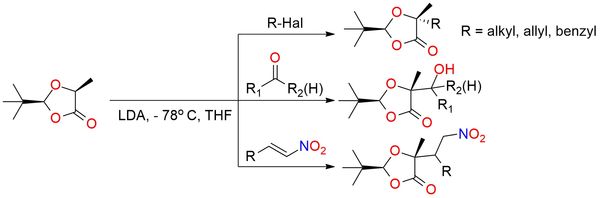
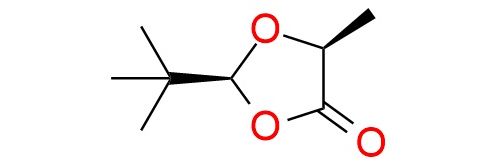
(S,S)-2-tert-Butyl-5-methyl-1,3-dioxolan-4-one is a chiral cyclic acetal derived from (S)-lactic acid and pivalaldehyde, commonly used as a precursor in asymmetric synthesis1. It serves as a reagent for the preparation of enantiopure α-hydroxy-α-methyl carboxylic acids through alkylation, hydroxyalkylation, and Michael addition reactions. Additionally, it can be used in radical reactions, Diels–Alder cycloadditions, and as a precursor to α,β-unsaturated carbonyl derivatives. Notable uses of the reagent include the synthesis of natural products and biologically active compounds such as Fronatalin, Feudomycinone C, and Eremantholid A. It is particularly valuable in reactions involving enolate formation, enabling highly diastereoselective transformations. The (S,S)-isomer is a stable solid under an inert atmosphere at low temperatures and soluble in common organic solvents.
Synonyms: (2S,5S)-2-tert-butyl-5-methyl-1,3-dioxolan-4-one; (2S,5S)-2-(tert-butyl)-5-methyl-1,3-dioxolan-4-one; 1,3-dioxolan-4-one, 2-(1,1-dimethylethyl)-5-methyl-, (2S,5S)-; 2-(s)-t-butyl-5-(s)-methyl-1,3-dioxolan-4-one
Selected publication
-
(R, R)-2-t-Butyl-5-Methyl-1,3-Dioxolan-4-One.
Sting A.; Seebach D.; Melgar-Fernández R.; Juaristi E. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/9780470842898.rb403m.pub2