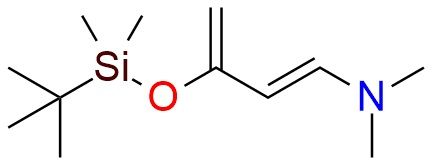
CAS 194233-66-4, Cat. No EN300-6503845
Reagent for cyclization

[(1E)-3-[(Tert-butyldimethylsilyl)oxy]buta-1,3-dien-1-yl]dimethylamine (Rawal’s diene) is a highly reactive diene designed to enhance Diels–Alder reactions. Compared to Danishefsky’s diene, Rawal’s diene shows significantly greater reactivity — up to 25–3000 times higher — especially with electron-deficient dienophiles, allowing cycloadditions to occur under much milder conditions (often at or below 0 °C)1. In general, any π-system activated by strong electron-withdrawing groups (carbonyl, sulfone, nitro, cyano, etc.) tends to undergo fast and high-yielding cycloaddition with Rawal’s diene. The combination of enamine and enol ether functionalities boosts both reactivity and regioselectivity. Rawal’s diene is used to synthesize pyranones, functionalized cyclohexanes, and complex natural products. There is a new protocol that enables easy and straightforward way to synthesize 2-alkyl-2,3-dihydro-4H-pyran-4-ones2. Recent advancements have made it available on a kilogram scale, with good stability under inert atmosphere and cold storage.
Synonyms: (1E)-3-[tert-butyl(dimethyl)silyl]oxy-N,N-dimethylbuta-1,3-dien-1-amine; 1-(dimethylamino)-3-(tert-butyldimethylsilyloxy)-1,3-butadiene; trans-3-(tert-butyldimethylsilyloxy)-N,N-dimethyl-1,3-butadien-1-amine; (E)-3-((tert-butyldimethylsilyl)oxy)-N,N-dimethylbuta-1,3-dien-1-amine; 3-((tert-butyldimethylsilyl)oxy)-N,N-dimethylbuta-1,3-dien-1-amine; [(1E)-3-[(tert-butyldimethylsilyl)oxy]buta-1,3-dien-1-yl]dimethylamine
Selected publications
-
Preparation and Diels−Alder Reactivity of 1-Amino-3-Siloxy-1,3-Butadienes.
Kozmin S. A.; Rawal V. H. J Org Chem 1997, 62 (16), 5252–5253. DOI: 10.1021/jo970438q
-
Shackles Off: A Kilo Scale Synthesis of Rawal’s Diene.
Shamrai O. I.; Iermolenko I. A.; Ostapchuk E. N.; Leha D. O.; Zarudnitskii E. V.; Ryabukhin S. V.; Volochnyuk D. M. Org Process Res Dev 2025. DOI: 10.1021/acs.oprd.5c00039