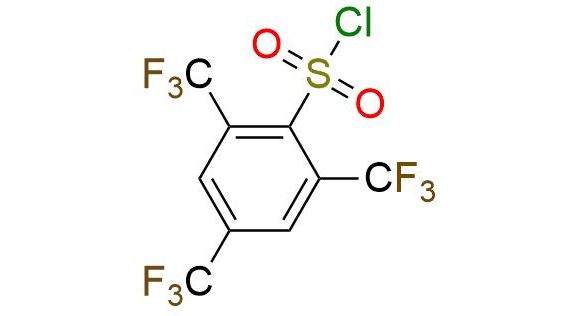
CAS 944453-80-9, Cat. No EN300-45671553
Protecting group for amines
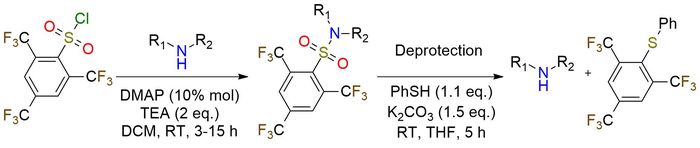
Nonafluoromesitylenesulfonyl (Nms) chloride is the new arenesulfonamide-based protecting reagent for primary and secondary amines1. It is a white crystalline solid, stable under ambient conditions and even in the presence of water. The Nms protection reaction can be conducted under mild conditions, delivering excellent yields when sulfonylating hindered amines. Notably, this protecting group demonstrates a broad functional-group tolerance, accommodating halides, boronic esters, nitro groups, carbonyls, and various heterocyclic structures. One key advantage of the Nms protecting group is its orthogonality to other benzenesulfonamides and the Boc protecting group. Additionally, unlike other benzenesulfonamide protecting groups (Ns, Cs, Ts), nonafluoromesitylenesulfonyl remains stable in the presence of reducing agents and organometallic reagents. Deprotection is performed in mild conditions and can be undergone without a base but with more equivalents of thiophenol.
Synonyms: NmsCl; 2,4,6-tris(trifluoromethyl)benzenesulfonyl chloride
Selected publication
-
Nms‐Amides: An Amine Protecting Group with Unique Stability and Selectivity.
Spieß P.; Sirvent A.; Tiefenbrunner I.; Sargueil J.; Fernandes A.; Arroyo‐Bondía A.; Meyrelles R.; Just D.; Prado‐Roller A.; Shaaban S.; Kaiser D.; Maulide N. Chemistry – A European Journal 2023, 29 (41). DOI: 10.1002/chem.202301312