CAS 76029-67-9, Cat. No EN300-18981232
Reagent for trifluoromethylation
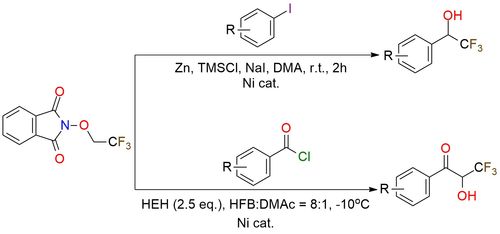
N-Trifluoroethoxyphthalimide is a redox-active reagent that generates α-hydroxytrifluoroethyl radicals. In nickel-catalyzed reductive cross-electrophile coupling with iodoarenes1, it provides α-aryl-α-trifluoromethyl alcohols in good yields (up to 88%) with broad scope, tolerating both electron-rich and electron-poor aryl iodides, halogens, esters, and ketones, enabling late-stage functionalization of bioactive scaffolds. Another methodology utilizes dual nickel/photoredox catalysis2 leading to enantioenriched α-trifluoromethyl acyloins (up to 95% yield, 92% ee), which can be reduced to anti-1,2-diols. The method is compatible with diverse acyl chlorides and complex drug-derived substrates, with stereocontrol arising from hydrogen-bonding in the transition state.
Synonyms: 2-(2,2,2-trifluoroethoxy)-1H-isoindole-1,3(2H)-dione; 2-(2,2,2-trifluoroethoxy)-2,3-dihydro-1H-isoindole-1,3-dione
Selected publications
-
Direct Synthesis of α-Aryl-α-Trifluoromethyl Alcohols via Nickel Catalyzed Cross-Electrophile Coupling.
Lombardi, L.; Cerveri, A.; Giovanelli, R.; Castiñeira Reis, M.; Silva López, C.; Bertuzzi, G.; Bandini, M. Angewandte Chemie - International Edition 2022, 61 (47). DOI: 10.1002/anie.202211732
-
Hydrogen Bonding Interaction Enabled Asymmetric C─H Acylation of Trifluoroethanol by Dual Nickel/Photoredox Catalysis.
Li, Y. B.; Hu, D. D.; Ren, W. R.; Liu, H.; Wang, Y. L.; Li, K.; Ke, W. C.; Jin, R. X.; Wang, X. S. Angewandte Chemie - International Edition 2025, 64 (21). DOI: 10.1002/anie.202424324