CAS 14204-24-1, Cat. No EN300-3505681
Reagent for sulfenylation
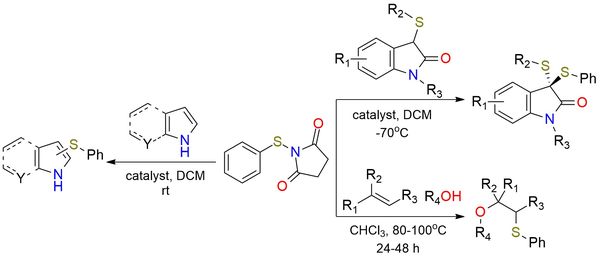
N-Thiophenylsuccinimide is a reagent commonly used in catalytic sulfenylation reactions. It demonstrated effectiveness in asymmetric sulfenylation for the synthesis of structurally diverse dithioketals1 and nitrogen-containing heterocycles2 under mild conditions. The scope of the methodology for dithioketals encompasses various sulfur-based nucleophiles, including 3-thiooxindoles and benzofuranone derivatives. The reagent also enables metal-free oxysulfenylation3 of alkenes in combination with alcohols to yield β-alkoxy sulfides. This approach offers a simple and efficient method that eliminates the need for catalysts, ligands, or additives, contributing to an environmentally friendly profile. The reaction exhibits broad functional group tolerance, including halogens, nitro-, ester-, and ether groups.
Synonyms: 1-(phenylthio)-2,5-pyrrolidinedione (ACI); succinimide, N-(phenylthio)- (6CI, 8CI); N-(phenylthio)succinimide; N-phenylsulfenylsuccinimide; N-thiophenylsuccinimide
Selected publications
-
Catalytic Asymmetric Sulfenylation to Structurally Diverse Dithioketals.
Zhou F.; Liao K.; Zhou J.; Yu J.; Gao W. Chemical Communications 2015, 51 (90), 16255–16258. DOI: 10.1039/c5cc07010d
-
A Conjugate Lewis Base-Brønsted Acid Catalyst for the Sulfenylation of Nitrogen Containing Heterocycles under Mild Conditions.
Nalbandian C.; Miller E.; Toenjes S.; Gustafson J. Chemical Communications 2017, 53 (9), 1494–1497. DOI: 10.1039/c6cc09998j
-
Metal-Free Oxysulfenylation of Alkenes with 1-(Arylthio)Pyrrolidine-2,5-Diones and Alcohols.
Yu J.; Gao C.; Song Z.; Yang H.; Fu H. Org Biomol Chem 2015, 13 (17), 4846–4850. DOI: 10.1039/c5ob00252d