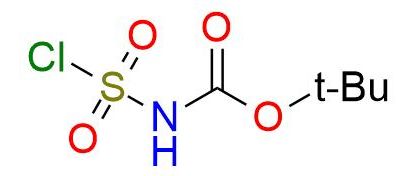
CAS 147000-89-3, Cat. No EN300-118173
Reagent for sulfamoylation
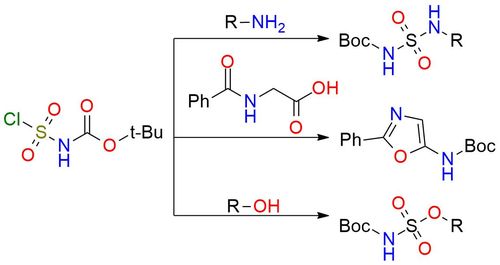
N-(tert-Butoxycarbonyl)sulfamoyl chloride is a highly versatile and reactive electrophilic sulfamoylating reagent with extensive use in organic synthesis. This white powder is soluble in numerous solvents and performs well in soft reaction conditions1. It readily reacts with nucleophiles, which leads to the formation of various sulfamates, sulfamides, hydroxysulfamides, and sulfonamides through reactions with alcohols, amine, hydroxylamines, and carbon-based nucleophiles2,3. One of the important advantages of the reagent compared to sulfamoyl chloride is the presence of a Boc protective group after the reaction. This minimizes the number of synthesis stages and facilitates the product purification process.
Synonyms: N-(tert-butoxycarbonyl)sulfamoyl chloride; tert-butyl N-(chlorosulfonyl)carbamate; tert-butyl chlorosulfonylcarbamate; N-Boc-sulfamoyl chloride
Selected publications
-
N-(Tert-Butoxycarbonyl)Sulfamoyl Chloride.
Dawadi S.; Aldrich C. Encyclopedia of Reagents for Organic Synthesis 2016, 1–3. DOI: 10.1002/047084289X.rn01906
-
The Discovery of N-[5-(4-Bromophenyl)-6-[2-[(5-Bromo-2-Pyrimidinyl)Oxy]Ethoxy]-4-Pyrimidinyl]- N′-Propylsulfamide (Macitentan), an Orally Active, Potent Dual Endothelin Receptor Antagonist.
Bolli M.; Boss C.; Binkert C.; Buchmann S.; Bur D.; Hess P.; Iglarz M.; Meyer S.; Rein J.; Rey M.; Treiber A.; Clozel M.; Fischli W.; Weller T. J Med Chem 2012, 55 (17), 7849–7861. DOI: 10.1021/jm3009103
-
Practical Asymmetric Synthesis of a Chiral Piperazinone Derivative.
McLaughlin M.; Belyk K.; Chen C.; Linghu X.; Pan J.; Qian G.; Reamer R. A.; Xu Y. Org Process Res Dev 2013, 17 (8), 1052–1060. DOI: 10.1021/op400150w