CAS 55962-05-5, Cat. No EN300-20000353
Reagent for N-transfer
![[N-(p-Toluenesulfonyl)imino]phenyliodinane](/images/Reagents/EN300-20000353_Scheme.jpg)
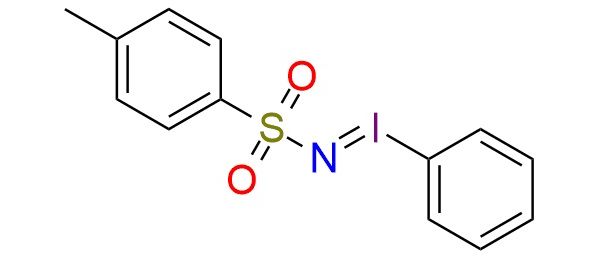
[N-(p-Toluenesulfonyl)imino]phenyliodinane is a hypervalent iodine(III) reagent used as a nitrene source for nitrogen-transfer reactions1. It enables aziridination of alkenes2, C–H bond amination, and heteroatom functionalization, often under metal catalysis (e.g., Cu, Mn, Fe, Pd). The reagent is effective in forming sulfoximines, iminosulfurane, phosphinimines, and N-tosylaziridines with high regio- and stereoselectivity. It is a stable, yellow solid that is safe to handle and commercially available. The reagent exhibits good functional group tolerance and can be used in asymmetric catalysis, including enantioselective aziridination.
Synonyms: 4-methyl-N-(phenyl-lambda3-iodanylidene)benzene-1-sulfonamide; (tosylimido)iodobenzene; (tosylimino)phenyliodinane; (tosyliminoiodo)benzene; 4-methyl-N-(phenyliodidene)benzenesulfonamide; [[(4-nethylphenyl)sulfonyl]imino]phenyliodinane; N-[(4-methylphenyl)sulfonyl]iminophenyliodinane; N-phenyliodoso-4-methylbenzenesulfonimide; N-tosyliminophenyliodinane; PhINTs
Selected publications
-
[N-(p-Toluenesulfonyl)Imino]Phenyliodinane.
Evans, D. A.; Barnes, D. M. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rt139
-
Practical, Fast, and High-Yielding Aziridination Procedure Using Simple Cu(II) Complexes Containing N-Donor Pyridine-Based Ligands.
Mohr, F.; Binfield, S. A.; Fettinger, J. C.; Vedernikov, A. N. A J Org Chem 2005, 70 (12), 4833–4839. DOI: 10.1021/jo050485f