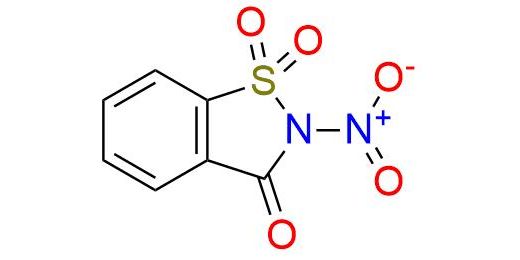
CAS 80283-99-4, Cat. No EN300-27164666
Reagent for nitration of organosilanes
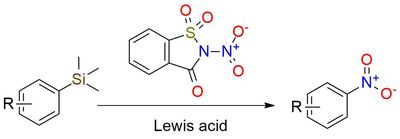
N-Nitrosaccharin, a recently introduced reagent, enables the facile, regio- and chemoselective nitration of (het)aryl silanes in Lewis acid presence1. It is a bench-stable white solid, and easy to work with. Methodology tolerates many functionalities such as halides, hydroxy, unprotected carboxylic acid, ketones, and formyl groups. Other heteroatom containing functional groups are admitted: pinacol boronate, imide, amide, carbamate, phosphonate, triflate, and sulfoxide1. Various electron-donating and electron-withdrawing aryl substituents at ortho-, meta-, and para-positions are well tolerated. The wide scope of reaction substrates includes 5- and 6-membered heterocycles. Some non-aromatic organosilanes such as alkenyl and alkynyl can also be nitrated with lower yields.
Synonyms: 1,2-benzisothiazolin-3-one, 2-nitro-, 1,1-dioxide (7CI); 2-nitro-1,1-dioxo-1,2-dihydro-1λ6-benzo[d]isothiazol-3-on
Selected publication
-
Catalytic Ipso‐Nitration of Organosilanes Enabled by Electrophilic N‐Nitrosaccharin Reagent.
Mosiagin I.; Fernandes A.; Budinská A.; Hayriyan L.; Ylijoki K.; Katayev D. Angewandte Chemie International Edition 2023, 62 (41). DOI: 10.1002/anie.202310851