CAS 1045822-31-8, Cat. No EN300-258021
Reagent for trifluoromethylthiolation
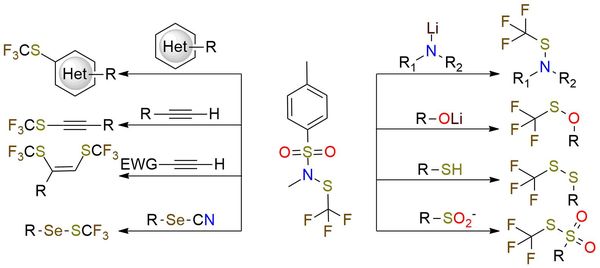
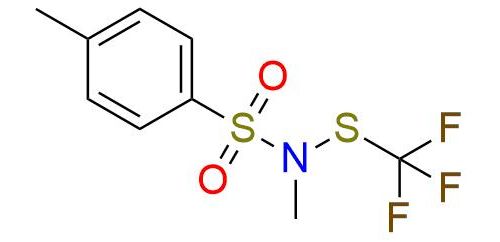
N-Methyl-N-tosyl trifluoromethanesulfenamide is a universal reagent for trifluoromethylthiolation of a broad scope of substrates1,2. It is yellow solid, bench-stable, and soluble in alkanes, alcohols, diethyl ether, dichloromethane, DMF, and DMSO2. The reagent reacts with heteroatomic nucleophiles, and organometallic reagents and can undergo aromatic electrophilic substitution with electron-rich aromatic and heteroaromatic compounds1. Furthermore, terminal alkynes reacting with it in copper-catalyzed, base-free conditions to yield trifluoromethylthiolated alkynes or, if an electron-withdrawing substituent is present to yield vic-bistrifluoromethylthiolated vinylic products with (Z)-selectivity1. The chemoselectivity of the reagent is controlled by the conditions of the reaction, which allows the usage of the reagent in the presence of many functional groups1, 2. The obtaining of trifluoromethanesulfenamides, including late-stage functionalization of bioactive compounds under mild conditions with iodide catalysis, is also described3.
Synonyms: benzenesulfonamide, N,4-dimethyl-N- [(trifluoromethyl)thio]; N,4-dimethyl-N-[(trifluoromethyl)sulfanyl] benzene-1-sulfonamide; BB23
Selected publications
-
Electrophilic Trifluoromethylthiolation of Carbonyl Compounds.
Alazet S.; Zimmer L.; Billard T. Chemistry – A European Journal 2014, 20 (28), 8589–8593. DOI: 10.1002/chem.201403409
-
Benzenesulfonamide, N,4-Dimethyl-N-[(Trifluoromethyl)Thio].
Billard T. Encyclopedia of Reagents for Organic Synthesis 2019, 1–3. DOI: 10.1002/047084289X.rn02269
-
CF3S-N bond formation under mild conditions. Easy access to trifluoromethanesulfenamides with a versatile reagent.
Delobel C.; Toulgoat F.; Billard T. Journal of Fluorine Chemistry 2024, 279, 110353. DOI: 10.1016/j.jfluchem.2024.110353