CAS 2439-83-0, Cat. No EN300-1717385
Reagent for bromination
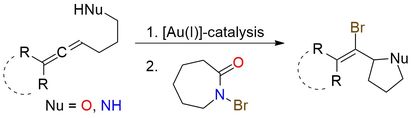
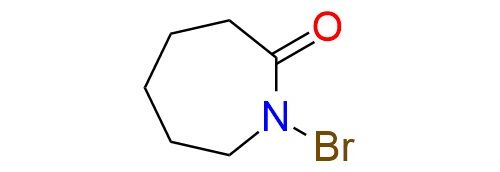
N-Bromocaprolactam is an electrophilic brominating reagent with broad applications in organic synthesis. Its scope includes the bromination of aromatic compounds, oxidation of alcohols to ketones, and selective bromination of isatins. Notably, it is a milder alternative to traditional brominating agents such as N-bromosuccinimide (NBS), as it does not require peroxide catalysts or actinic light to initiate bromination reactions1. In gold-catalyzed reactions, it participates as a brominating agent for bromocyclization reactions of allenes, yielding enantioenriched vinyl bromides suitable for further transformations like cross-coupling2,3. N-Bromocaprolactam is a stable crystalline solid, melting at 64–66°C, and compatible with a variety of solvents and conditions.
Synonyms: 1-bromoazepan-2-one; 1-bromohexahydro-2H-azepin-2-one; N-bromo-caprolactam; N-vromo-6-hexanolactone; 1-bromohexahydro-2H-azepine-2-one; 2H-azepin-2-one, 1-bromohexahydro-
Selected publications
-
Notes- N-Bromocaprolactam
Taub B.; Hino J. J Org Chem 1960, 25 (2), 263–264. DOI: 10.1021/jo01072a604
-
Gold(I)-Catalyzed Enantioselective Bromocyclization Reactions of Allenes.
Miles D.; Veguillas M.; Toste F. Chem Sci 2013, 4 (9), 3427–3431. DOI: 10.1039/c3sc50811k
-
Enantioselective Halofunctionalization of Alkenes.
Ashtekar K.; Jaganathan A.; Borhan B.; Whitehead D. Organic Reactions 2021, 105, 1–266. DOI: 10.1002/0471264180.or105.01