CAS 105838-14-0, Cat. No EN300-7432200
Reagent for amination
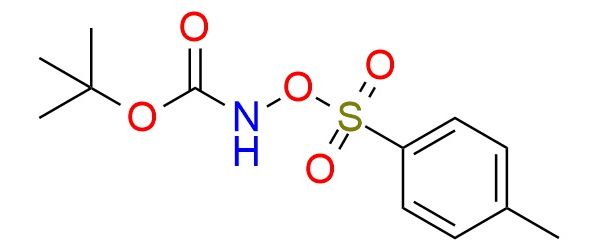
N-Boc-O-tosylhydroxylamine is a stable, crystalline reagent used as an electrophile and particularly effective for direct amination of carbonyl compounds, active methylene compounds, and enolates, enabling α-amination under mild conditions1. The Boc protecting group enhances stability and selectivity, while the tosyl leaving group ensures good reactivity. The reagent shows broad functional group tolerance, allowing amination in the presence of esters, nitriles, and aromatic substituents without significant side reactions. Reported applications include amination of β-keto esters, α-amination in heterocycle synthesis, and preparation of protected α-amino acids2,3. It is typically handled under dry conditions and stored at low temperature to avoid decomposition.
Synonyms: TsONHBoc; N-boc-O-tosyl hydroxylamine; tert-butyl N-tosyloxycarbamate; tert-butyl {[(4-methylphenyl)sulfonyl]oxy}carbamate; tert-butyl ([(4-methylphenyl)sulfonyl]oxy)carbamate; t-butyl-n-tosyloxycarbamate; (tert-butoxycarbonylamino) 4-methylbenzenesulfonate; tert-butyl [(4-methylphenyl)sulfonyl]oxycarbamate; tert-butyl N-[(4-methylbenzenesulfonyl)oxy]carbamate
Selected publications
-
N‐Boc‐O‐tosylhydroxylamine.
Lattanzi, A.; Adams, R. J.; Mudd, R. J.; Bower, J. F. Encyclopedia of Reagents for Organic Synthesis 2025, 1–6. DOI: 10.1002/047084289X.rn01753.pub2
-
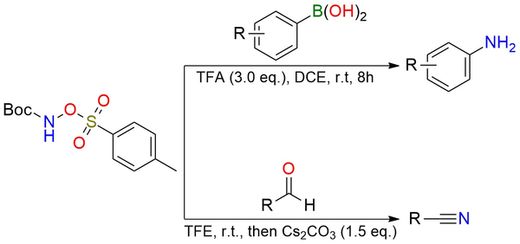
Metal-Free Direct Transformation of Aryl Boronic Acid to Primary Amines.
Kumar, P.; Verma, S.; Rathi, K.; Chandra, D.; Prakash Verma, V.; Jat, J. L. European J Org Chem 2022, 2022 (27). DOI: 10.1002/ejoc.202200508
-
Metal-Free Synthesis of Nitriles from Aldehydes Using N-Boc-O-Tosylhydroxylamine as a Nitrogen Source.
Kumar, P.; Singh, V.; Jat, J. L.; Tiwari, B. New Journal of Chemistry 2022, 47 (2), 535–538. DOI: 10.1039/d2nj04595h