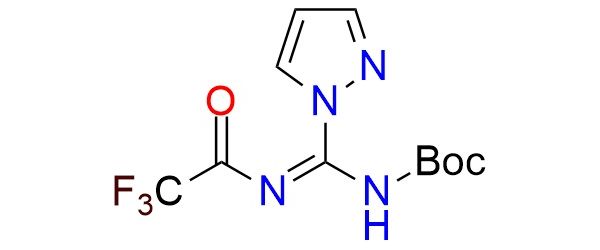
CAS 2028307-97-1, Cat. No EN300-22108725
Reagent for guanidinylation
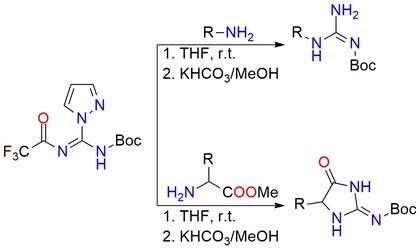
N-Boc-N′-TFA-pyrazole-1-carboxamidine is a highly efficient guanidinylation reagent1. It reacts with amines, including sterically hindered and electron-deficient ones, to form N-Boc-substituted guanidines under mild conditions. After the initial guanidinylation, the trifluoroacetyl group is readily removed under basic conditions, yielding free guanidine derivatives. The reagent is particularly valuable in synthesizing guanidine-containing heterocycles, e.g., in the total synthesis of araiosamines. It also reacts with amino acid derivatives to form intermediates that undergo intramolecular cyclization, affording bis-acyl guanidines in high yields. It is a colorless solid, soluble in common organic solvents, and is typically stored at −20 °C to ensure stability.
Synonyms: tert-butyl (NE)-N-[pyrazol-1-yl-[(2,2,2-trifluoroacetyl)amino]methylidene]carbamate; tert-butyl (Z)-((1H-pyrazol-1-yl)((2,2,2-trifluoroacetyl)imino)methyl)carbamate; N-Boc-N′-TFA-pyrazole-1-carboxamidine; tert-butyl N-[(1H-pyrazol-1-yl)[(2,2,2-trifluoroacetyl)imino]methyl]carbamate
Selected publication
-
Tert‐Butyl (Z)‐((1-H tert-Butyl (Z)-((1H-pyrazol-1-yl)((2,2,2-Trifluoroacetyl)imino)methyl)carbamate.
Lebel, H. Encyclopedia of Reagents for Organic Synthesis 2021, 1–1. DOI: 10.1002/047084289X.rn02315