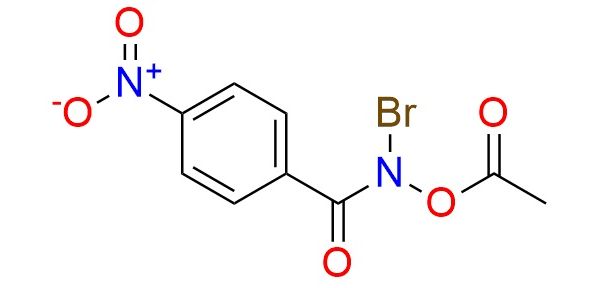
CAS 2991507-63-0, Cat. No EN300-46605562
Reagent for bromination
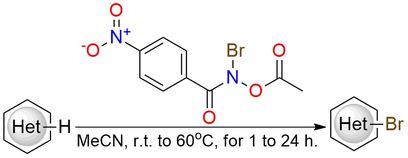
N-Acetoxy-N-bromo-4-nitrobenzamide is a bench-stable, crystalline reagent enabling site-selective C–H bromination under mild, metal-free conditions1. Designed with an electron-deficient aryl backbone and an acetate leaving group, it ensures controlled and selective bromine transfer. The reagent exhibits excellent functional group tolerance and high chemoselectivity even in the presence of potentially reactive motifs such as alcohols, amides, and alkenes. Notably, it favors bromination of electron-rich aromatic rings while avoiding overbromination or side reactions. The selectivity extends to late-stage functionalization of complex molecules, including pharmaceuticals and natural products. N-Acetoxy-N-bromo-4-nitrobenzamide performance surpasses many traditional electrophilic brominating agents in terms of safety, predictability, and compatibility with sensitive functional groups.
Synonyms: N-bromo-1-(4-nitrophenyl)formamido acetate; N-acetoxy-N-bromo-4-nitrobenzamide
Selected publication
-
Discovery of N–X Anomeric Amides as Electrophilic Halogenation Reagents.
Wang, Y.; Bi, C.; Kawamata, Y.; Grant, L. N.; Samp, L.; Richardson, P. F.; Zhang, S.; Harper, K. C.; Palkowitz, M. D.; Vasilopoulos, A.; Collins, M. R.; Oderinde, M. S.; Tyrol, C. C.; Chen, D.; LaChapelle, E. A.; Bailey, J. B.; Qiao, J. X.; Baran, P. S. Nat Chem 2024, 16 (9), 1539–1545. DOI: 10.1038/s41557-024-01539-4