CAS 82911-69-1, Cat. No EN300-68359
Reagent for amino group protection
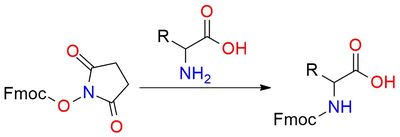
N-(9-Fluorenylmethyloxycarbonyl)oxysuccinimide (Fmoc-OSu) is a reagent widely used for introducing the Fmoc protecting group to amines, a key step in peptide synthesis1. Its scope includes selective Fmoc protection in amino acids and various amine-containing compounds. The reagent offers high stability against acid, allowing compatibility with other protective groups like Boc and Cbz. The deprotection is efficiently achieved using bases such as piperidine, which removes the Fmoc group without hydrolysis. Fmoc-OSu is a white solid soluble in organic solvents such as THF, DMF, and dichloromethane, and should be stored dry.
Synonyms: N-(9-fluorenylmethyloxycarbonyl)oxysuccinimide; Fmoc-OSu; Fmoc N-hydroxysuccinimide ester; 9-fluorenylmethylsuccinimidyl carbonate; N-(Fmoc-oxy)succinimide
Selected publication
-
N-(9-Fluorenylmethyloxycarbonyl)Oxysuccinimide.
Raillard S. Encyclopedia of Reagents for Organic Synthesis 2002. DOI: 10.1002/047084289X.rn00142