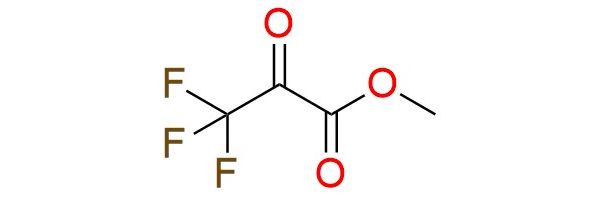
CAS 13089-11-7, Cat. No EN300-42930
Reagent for cyclization and Friedel–Crafts alkylation
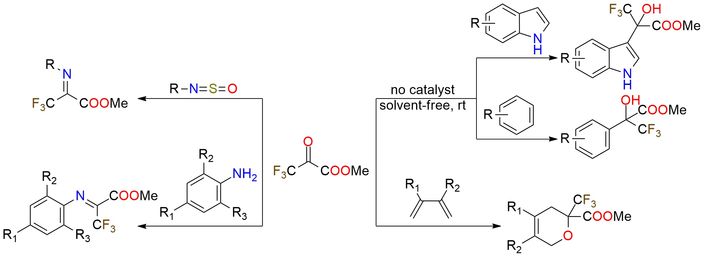
Methyl trifluoropyruvate is a highly electrophilic reagent due to the strong electron-withdrawing trifluoromethyl group adjacent to the keto functionality. It is widely used in Friedel–Crafts alkylation, aldol condensations, cycloadditions, and carbon–nucleophile additions1. The reagent reacts smoothly with aromatic and heteroaromatic compounds under mild or catalyst-free conditions, often with excellent yields. High reactivity enables reagent usage in asymmetric catalysis, imine formation, fluorinated amino acids and heterocycles synthesis. Methyl trifluoropyruvate is an air-sensitive and thermally stable colorless to yellow liquid that should be stored away from oxidizers.
Synonyms: methyl 3,3,3-trifluoro-2-oxopropanoate; methyl 3,3,3-trifluoropyruvate; trifluoropyruvic acid methyl ester; propanoic acid, 3,3,3-trifluoro-2-oxo-, methyl ester; methyl 2-oxo-3,3,3-trifluoropropanoate; methyltrifluoropyruvate; pyruvic acid, trifluoro-methyl ester
Selected publication
-
Methyltrifluoropyruvate.
Figueroa, R.; Hsung, R. P.; Li, G.; Yang, J. H. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rn00769