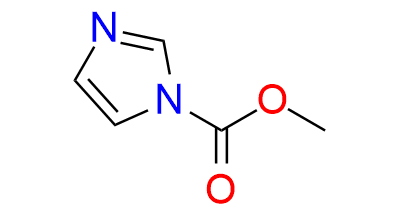
CAS 61985-23-7, Cat. No EN300-7613220
Reagent for esterification and amidation
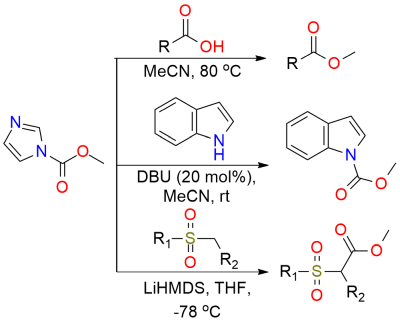
Methyl imidazole carbamate (MImC) is a reagent for chemoselective esterification of carboxylic acids[1]. It offers a safer and more efficient alternative to traditional methods like diazomethane. MImC operates well in polar solvents (e.g. MeCN or EtOAc) and at elevated temperatures, achieving high yields and selectivity. MImC is particularly useful for carboxylic acid esterification with minimal impact on other functional groups, making it a valuable tool for complex molecule synthesis and late-stage functionalization. Despite its effectiveness, care must be taken with substrates that may undergo racemization, like carboxylic acids with epimerizable stereocenters. The next limit - is a substrate with highly acidic phenols that can impede the esterification. Besides, the reagent is effective in the N-acylation of indoles and oxazolidinones[2] or crucial in the synthesis of alkoxycarbonyl benzo[d]thiazol sulfones[3].
Synonyms: MImC; Sarpong reagent; Imidazole-1-carboxylic acid, methyl ester; methyl imidazole-1-carboxylate; Methyl 1H-imidazole-1-carboxylate; 1H-Imidazole-1-carboxylic acid, methyl ester (9CI); 1-methoxycarbonylimidazole; 1-methoxycarbonyl imidazole
Selected publication
-
Chemoselective Esterification and Amidation of Carboxylic Acids with Imidazole Carbamates and Ureas.
Heller S. T.; Sarpong R. Org Lett 2010, 12 (20), 4572–4575. DOI: 10.1021/ol1018882
-
Chemoselective N-Acylation of Indoles and Oxazolidinones with Carbonylazoles.
Heller S. T.; Schultz E. E.; Sarpong R. Angewandte Chemie - International Edition 2012, 51 (33), 8304–8308. DOI: 10.1002/anie.201203976
-
Practical Synthesis of β-Oxo Benzo[d]Thiazolyl Sulfones: Scope and Limitations.
Pospíil J.; Robiette R.; Sato H.; Debrus K. Org Biomol Chem 2012, 10 (6), 1225–1234. DOI: 10.1039/c1ob06510f