CAS 24225-08-9, Cat. No EN300-85890
Reagent for cyanation and cycloaddition
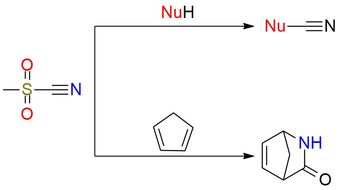
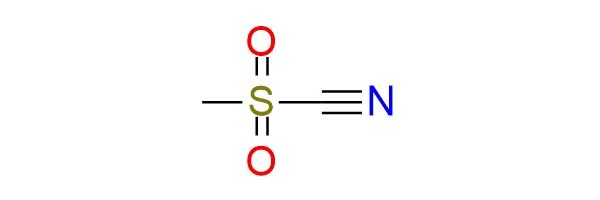
Methanesulfonyl cyanide (MsCN) is a more reactive and more unstable analogue of tosyl cyanide (TsCN), known as a strong electrophilic cyanating reagent1. It is a volatile liquid/low-melting solid that is shelf-stable for a prolonged period without loss of purity and can be distilled without decomposition2. MsCN cleanly transfers the CN group to many nucleophiles (phenoxides, amines, thiols, Grignard reagents, organolithiums, cuprates, and enolates), giving cyanates in high yields. It is also a reactive dienophile and dipolarophile, participating in [4+2] cycloadditions and 1,3-dipolar cycloadditions to form pyrimidines, oxadiazoles, triazoles, tetrazoles, and other heterocycles. Due to its reactivity, MsCN is usually applied on a small scale and in cases when TsCN fails to yield results.
Synonyms: methanesulphonyl cyanide; methylsulfonyl cyanide; MsCN
Selected publications
-
Photoinduced Copper‐Catalyzed Asymmetric Radical Sulfonylcyanation of Vinylarenes with Sulfonyl Cyanides.
Zhao, K.; Zhang, B.; Zhao, F.; Xiao, W.; Chen, J. ChemCatChem 2025, 17 (12). DOI: 10.1002/cctc.202500258
-
P-Toluenesulfonyl Cyanide.
Griffiths, G. J.; Markiewicz, J. T. Encyclopedia of Reagents for Organic Synthesis 2014, 1-5. DOI: 10.1002/047084289X.rt136m.pub2