CAS 3534-25-6, Cat. No EN300-45159795
Protecting group for boronic acids
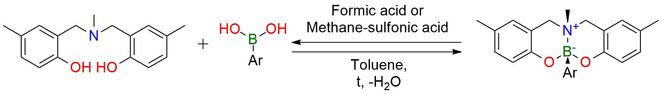
MeBOMA is a new protecting group (PG) for boronic acids1. MeBOMA-protected boronic acids have shown to be shelf-stable compounds that are solid, soluble in the THF and chloroform, and survive at high temperatures (150℃ in a vacuum). MeBOMA PG is stable under neutral and base conditions and has prolonged stability in soft acidic conditions at lower temperatures (<20℃), but at higher temperatures, it is deprotected. MeBOMA masks the boronates effectively during the Suzuki−Miyaura reaction, even under aqueous basic conditions, allowing for selective cross-coupling. MeBOMA tolerates the cleavage of a methyl ester to form carboxylic acid (basic conditions) and strong nucleophilic organometallic bases such as n-BuLi, allowing lithium−halogen exchange reactions at -78℃ in high yield. MeBOMA-protected boronates were stable to hydroxydeboronation with peroxides under conditions that convert most boronic groups into corresponding phenols.
Synonyms: 2-({[(2-hydroxy-5-methylphenyl)methyl](methyl)amino}methyl)-4-methylphenol; 2,2′-[(methylimino)bis(methylene)]bis[4-methylphenol]
Selected publication
-
Protection of Boronic Acids Using a Tridentate Aminophenol ONO Ligand for Selective Suzuki–Miyaura Coupling.
Simon P. M.; Castillo J. O.; Owyong T. C.; White J. M.; Saker Neto N.; Wong W. W. H. J Org Chem 2023, 88 (3), 1590–1599. DOI: 10.1021/acs.joc.2c02651