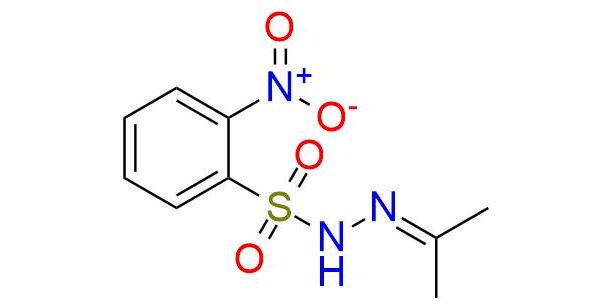
CAS 6655-27-2, Cat. No EN300-7423026
Reagent for synthesis of allenes and reductive transposition
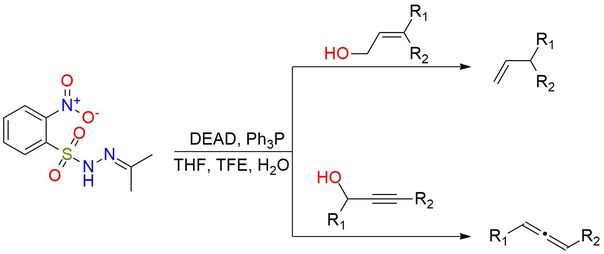
IPNBSH is a reagent with a unique niche and is generally used in organic synthesis for the preparation of allenes from propargylic alcohols, reductive transposition of allylic alcohols, and allylic bromides1. The reagent is known for its efficient ability to deoxygenate unhindered alcohols2. The reagent is stable at ambient temperature for several months. It is a white solid, soluble in THF, DMSO, acetone, acetonitrile, and DMF. The reaction conditions are mild, typical for Mitsunobu reactions, and generally occur with a good yield.
Synonyms: N-isopropylidene N-2-nitrobenzenesulfonyl hydrazine; isopropylidene o-nitrobenzenesulfonyl hydrazide
Selected publication
-
Benzenesulfonic Acid, 2-Nitro-, (1-Methylethylidene)Hydrazide.
Movassaghi M.; Ahmad O. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rn00925
-
N-Isopropylidene-N‘-2-Nitrobenzenesulfonyl Hydrazine, a Reagent for Reduction of Alcohols via the Corresponding Monoalkyl Diazenes.
Movassaghi M.; Ahmad O. J Org Chem 2007, 72 (5), 1838–1841. DOI: 10.1021/jo062325p