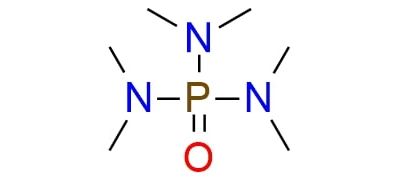
CAS 680-31-9, Cat. No EN300-17081
Reagents with superb ability to form cation–ligand complexes
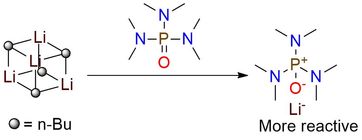
HMPA is among the strongest electron pair donors and is superior to protic solvents in cation solvating1. This coordinating ability allows enhancement of the rates of a wide variety of organolithium reactions and significantly influences regio- and stereochemistry1. The reagent is soluble well in polar and nonpolar solvents and is an essential part of enolate stereochemistry and ylide reactivity1. Besides, HMPA addition enables the formation of lithium enolates with substrates that cannot be metalated by LDA.
Synonyms: hexamethylorthophosphoric triamide; hexamethylphosphoramide; hexamethylphosphoric acid triamide; hexamethylphosphoric triamide; hexamethylphosphorotriamide; hexamethyltriamidophosphate; phosphoric acid hexamethyltriamide; phosphoric hexamethyltriamide; phosphoric tris(dimethylamide); phosphoryl hexamethyltriamide; tris(dimethylamino)phosphine oxide
Selected publication
-
Hexamethylphosphoric Triamide.
Dykstra R. R. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rh020