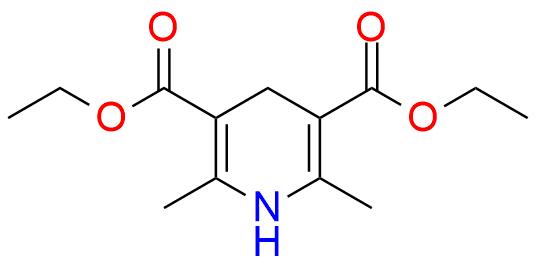
CAS 1149-23-1, Cat. No EN300-26986
Reagent the source of hydride
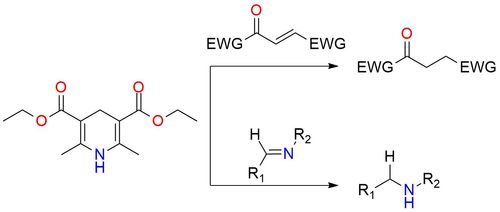
Hantzsch ester is a mild, stoichiometric, nonmetallic reducing agent and a classical hydride source for various reduction reactions. It is solid, bench-stable, and soluble in most organic solvents1. The activated C=C bonds, C=O bonds in carbonyl compounds, and C=N bonds in imines are the substrates in reactions with Hantzsch ester. The usage of chiral catalysts in combination with this reducing agent leads to enantioselective hydride transfer1.
Synonyms: 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid diethyl ester; 1,4-dihydro-3,5-dicarboxylic acid diethyl ester-2,6-dimethylpyridine; 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid diethyl ester; 2,6-dimethyl-3,5-di(carboethoxy)-1,4-dihydropyridine; 2,6-dimethyl-3,5-dicarbethoxy-1,4-dihydropyridine; 2,6-dimethyl-3,5-diethoxycarbonyl-1,4-dihydropyridine; 3,5-bis(ethoxycarbonyl)-1,4-dihydro-2,6-dimethylpyridine; 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine; 3,5-dicarbethoxy-2,6-dimethyl-1,4-dihydropyridine; HEH; Hantzsch ester hydride; Hantzsch dihydropyridine
Selected publication
-
Diethyl 1,4-Dihydro-2,6-Dimethyl-3,5-Pyridinedicarboxylate.
Bechara W. S.; Charette A. B.; Na R.; Wang W.; Zheng C. Encyclopedia of Reagents for Organic Synthesis 2020, 1–12. DOI: 10.1002/047084289X.rn01318.pub2