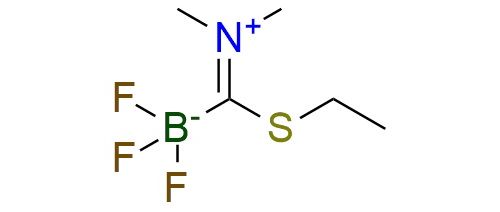
CAS 1622923-36-7, Cat. No EN300-19465421
Reagent for KATs synthesis
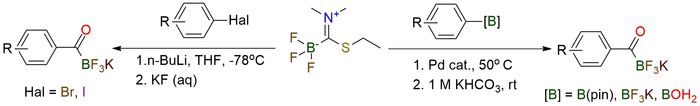
(Ethylthio-trifluoroborate)-methane dimethyliminium is a novel and highly efficient reagent developed for the synthesis of potassium acyltrifluoroborates (KATs). Its scope includes the preparation of KATs from aryl and heteroaryl halides via a one-step process involving organolithium intermediates1. This protocol enables the synthesis of KATs containing a variety of functional groups, including pyridines, esters, nitro groups, and halides, overcoming limitations of previous methods that required harsh conditions or multistep syntheses. Another protocol enables efficient preparation of KATs from boronic acids, pinacol esters, or potassium organotrifluoroborats via Pd-catalyzed cross-coupling2. This method tolerates a broad range of functional groups, including esters, halides, and nitriles, and provides high yields under mild aqueous conditions. In terms of stability, the reagent is air-stable and can be stored for extended periods. It is compatible with many organic solvents, making it practical for large-scale applications.
Synonyms: [(dimethyliminiumyl)(ethylsulfanyl)methyl]trifluoroboranuide
Selected publication
-
A Reagent for the One-Step Preparation of Potassium Acyltrifluoroborates (KATs) from Aryl- and Heteroarylhalides.
Erös G.; Kushida Y.; Bode J. Angewandte Chemie - International Edition 2014, 53 (29), 7604–7607. DOI: 10.1002/anie.201403931
-
Catalytic Synthesis of Potassium Acyltrifluoroborates (KATs) from Boronic Acids and the Thioimidate KAT Transfer Reagent.
Schuhmacher A.; Ryan S.; Bode J. Angewandte Chemie - International Edition 2021, 60 (8), 3918–3922. DOI: 10.1002/anie.202014581