CAS 1099-45-2, Cat. No EN300-19609
Stabilized phosphorane reagent useful for Wittig alkenation reactions
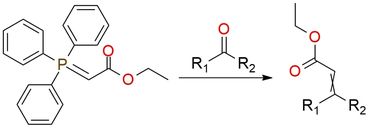
Ethyl (triphenylphosphanylidene)acetate, recognized as a versatile reagent, primarily serves as a reagent in Wittig alkenation reactions. This white crystalline powder maintains shelf stability and demonstrates favorable solubility in chloroform and similar solvents while displaying limited solubility in polar solvents such as water and alcohols. Except for the Witting reaction, this reagent is found to be used in heterocycle synthesis. Different ring systems can be prepared using stabilized phosphoranes with ylide and ester reactive sites. The reagent's nucleophilicity allows it to undergo alkylation with reactive electrophiles, generating phosphorus reagents amenable to subsequent transformations. The ylide can be halogenated or sulfonated to prepare more complex ylides, which can then undergo Wittig reactions to yield vinyl halides or vinyl sulfides, respectively1.
Synonyms: acetic acid, (triphenylphosphoranylidene)-, ethyl ester (6CI, 8CI, 9CI); (2-ethoxy-2-oxoethylidene)triphenylphosphorane; (carbethoxymethylene)triphenylphosphoran; (carbethoxymethylene)triphenylphosphorane; (carbethoxymethylidene)triphenylphosphorane; (ethoxycarbonylmethylidene)triphenylphosphorane; (triphenyl-λ5-phosphanylidene)acetic acid ethyl ester; (triphenylcarbethoxymethylene)phosphorane; (triphenylphosphanylidene)acetic acid ethyl ester; (triphenylphosphoranylidene)acetic acid ethyl ester; [(ethoxycarbonyl)methylene]triphenylphosphorane; carbethoxytriphenylphosphonium methylide
Selected publication
-
(Ethoxycarbonylmethylene)Triphenylphosphorane.
Reitz A. B.; McDonnell M. E.; Nikonov G. Encyclopedia of Reagents for Organic Synthesis 2012. DOI: 10.1002/047084289X.re023.pub2

