CAS 1242990-43-7, Cat. No EN300-43379233
Reagent for Horner–Emmons reaction
![Ethyl 2-[(tert-butyldimethylsilyl)oxy]-2-(diethoxyphosphoryl)acetate](/images/Reagents/EN300-43379233_Scheme.jpg)
The reagent is a silyl-protected phosphonate used to synthesize α-siloxy α,β-unsaturated esters via Horner–Emmons reactions. These esters act as masked α-ketoesters, which can be unmasked under acidic conditions for further transformations1,2. The reagent enables efficient synthesis of β-carbolines through Pictet–Spengler reactions and is applicable in the preparation of complex bioactive molecules, such as yohimbine and tadalafil3. It offers high enantioselectivity, a broad substrate scope, and avoids the handling of unstable intermediates. The versatility of the reagent makes it valuable for modular and scalable heterocycle synthesis.
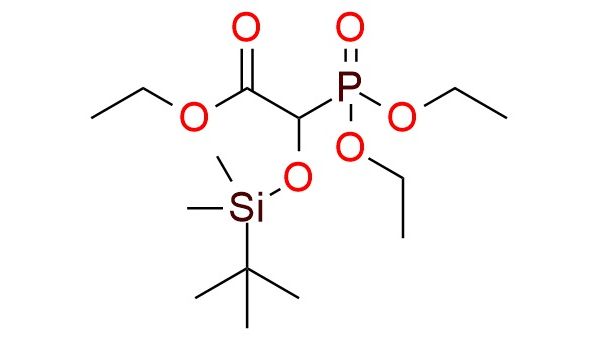
Synonyms: ethyl 2-[(tert-butyldimethylsilyl)oxy]-2-(diethoxyphosphoryl)acetate; acetic acid, 2-(diethoxyphosphinyl)-2-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, ethyl ester
Selected publications
-
A Convenient Method for the Synthesis of α-Ketoesters from Aldehydes.
Horne, D.; Gaudino, J.; Thompson, W. J. Tetrahedron Lett 1984, 25 (33), 3529–3532. DOI: 10.1016/S0040-4039(01)91067-6
-
New Acyl Anion Equivalent. A Short Route to the Enol Lactam Intermediate in Cytochalasin Synthesis.
Nakamura, E. Tetrahedron Lett 1981, 22 (7), 663–666. DOI: 10.1016/S0040-4039(01)92517-1
-
Synthesis of β-Carbolines from Aldehydes and Ketones via the α-Siloxy α,β-Unsaturated Esters.
He, S.; Lai, Z.; Yang, D. X.; Hong, Q.; Reibarkh, M.; Nargund, R. P.; Hagmann, W. K. Tetrahedron Lett 2010, 51 (33), 4361–4364. DOI: 10.1016/j.tetlet.2010.06.053