CAS 17507-56-1, Cat. No EN300-22046812
Reagent carbene precursor
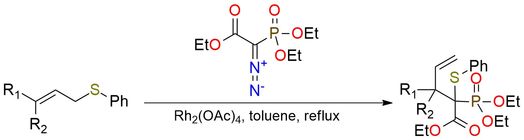
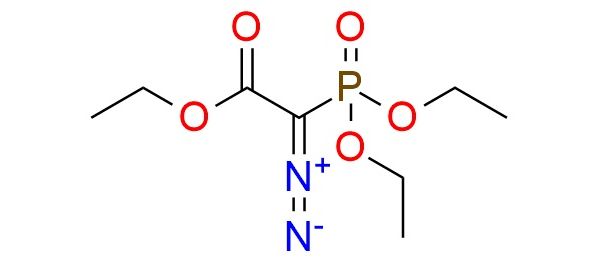
Ethyl 2-diazo-2-(diethoxyphosphinyl)acetate is an α-diazo phosphonate used as a carbene precursor in Rh(II)-catalyzed transformations1. It can be applied in [2,3]-sigmatropic rearrangements of allyl sulfides to γ,δ-unsaturated carbonyl compounds in high yields (often >90%), with broad scope including aromatic, heteroaromatic, aliphatic, and functionalized allyl sulfides2. The reaction shows high chemoselectivity, avoiding cyclopropanation and other side products, and tolerates electron-donating, electron-withdrawing, and sterically hindered groups. It is also compatible with sensitive functionalities such as esters, halides, and protected alcohols, making it valuable for multi-step synthesis.
Synonyms: ethyl 2-diazo-2-diethoxyphosphoryl-acetate; acetic acid, diazo(diethoxyphosphinyl)-, ethyl ester; acetic acid, diazophosphono-, triethyl ester; ethyl 2-diazo-2-(diethoxyphosphinyl)acetate; ethyl 2-diazo-2-(diethoxyphosphoryl)acetate; triethyl diazophosphonoacetate
Selected publications
-
An Improved and Efficient Method for Diazo Transfer Reaction of Active Methylene Compounds.
Lee, J. C.; Yuk, J. Y. Synth Commun 1995, 25 (10), 1511–1515. DOI: 10.1080/00397919508011762
-
Efficient Route to γ,δ-Unsaturated Carbonyl Compounds from Allyl Sulfides and α-Diazocarbonyls Using a Rhodium Catalyst.
Takano, S.; Tomita, S.; Takahashi, M.; Ogasawara, K. Chem Lett 1987, 16 (8), 1569–1570. DOI: 10.1246/cl.1987.1569