CAS 135654-49-8, Cat. No EN300-35573109
Reagent for сyclization and fluoroalkylation
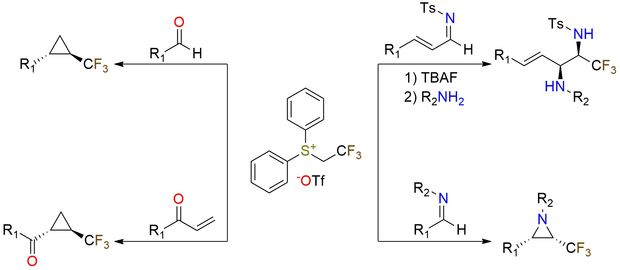
Diphenyl(2,2,2-trifluoroethyl)sulfonium triflate is a stable CF3-transfer reagent that forms sulfonium ylides under fluoride activation1. It enables the epoxidation of aldehydes and the cyclopropanation of vinyl ketones with excellent trans-selectivity, and the aziridination of imines with cis-selectivity. These aziridines can be opened with amines or alkoxides in one pot to give CF3-vicinal diamines (syn-selective) and CF3-amino alcohols2. The method tolerates vinyl and aryl imines, as well as electron-rich and electron-poor aryl and alkyl amines. This reagent offers a mild and convenient route to valuable CF3-functionalized building blocks, featuring a broad scope and strong stereocontrol.
Synonyms: sulfonium, diphenyl(2,2,2-trifluoroethyl)-, 1,1,1-trifluoromethanesulfonate (1:1); diphenyl(2,2,2-trifluoroethyl)sulfanium trifluoromethanesulfonate
Selected publications
-
Diastereoselective Johnson-Corey-Chaykovsky Trifluoroethylidenation.
Duan, Y.; Zhou, B.; Lin, J. H.; Xiao, J. C. Chemical Communications 2015, 51 (66), 13127–13130. DOI: 10.1039/c5cc04991a
-
Diastereoselective Synthesis of CF3-Containing Vicinal Diamines.
Huang, Q. X.; Zheng, Q. T.; Duan, Y.; Lin, J. H.; Xiao, J. C.; Zheng, X. Journal of Organic Chemistry 2017, 82 (15), 8273–8281. DOI: 10.1021/acs.joc.7b01261

